Amemori Group
Neuroscience, Cognitive Neurophysiology
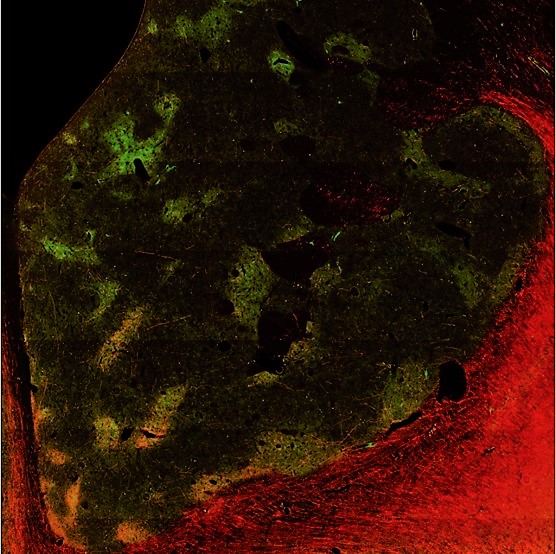
Research Overview
Control of Psychiatric Disease in Genetically Modified Primate Brain
A fundamental goal of human psychiatry is to guide and control emotion and anxiety. Despite its importance, we have not yet elucidated how the brain generates mood and anxiety disorders. Currently, for the treatment of anxiety disorder, obsessive-compulsive disorder and depression, drug medication has been selected, regardless of its severity. Clinical trials of deep brain stimulation acting on the local circuit have started, but the target exists widely in the limbic system, making a systematic understanding remain difficult. It is thus critically important to perform a systematic study to examine the function of the emotional circuit in the non-human primates (NHPs), whose brain structure is homologous to humans.
Many of our decisions involve weighing benefits against costs. The approach-avoidance conflict is an essential psychological concept and has been used to characterize anxiety-like behavior and pessimistic decision-making. An optimistic view leads to more approach decisions, and a pessimistic view leads to more avoidance decisions. Decisions under approach-avoidance conflict have primarily contributed to characterize the effect of various types of anti-anxiety drugs. With this task, we could successfully quantify how the subject evaluates the value of reward and punishment. We found that manipulating the primate pregenual anterior cingulate cortex (pACC) specifically influenced the balance between reward and punishment. We have shown that the pACC region preferentially sends a signal to the striosome compartment in the striatum(Figure 1). We will contribute to the construction of behavioral assays for the ASHBi community, producing genetically engineered NHPs.
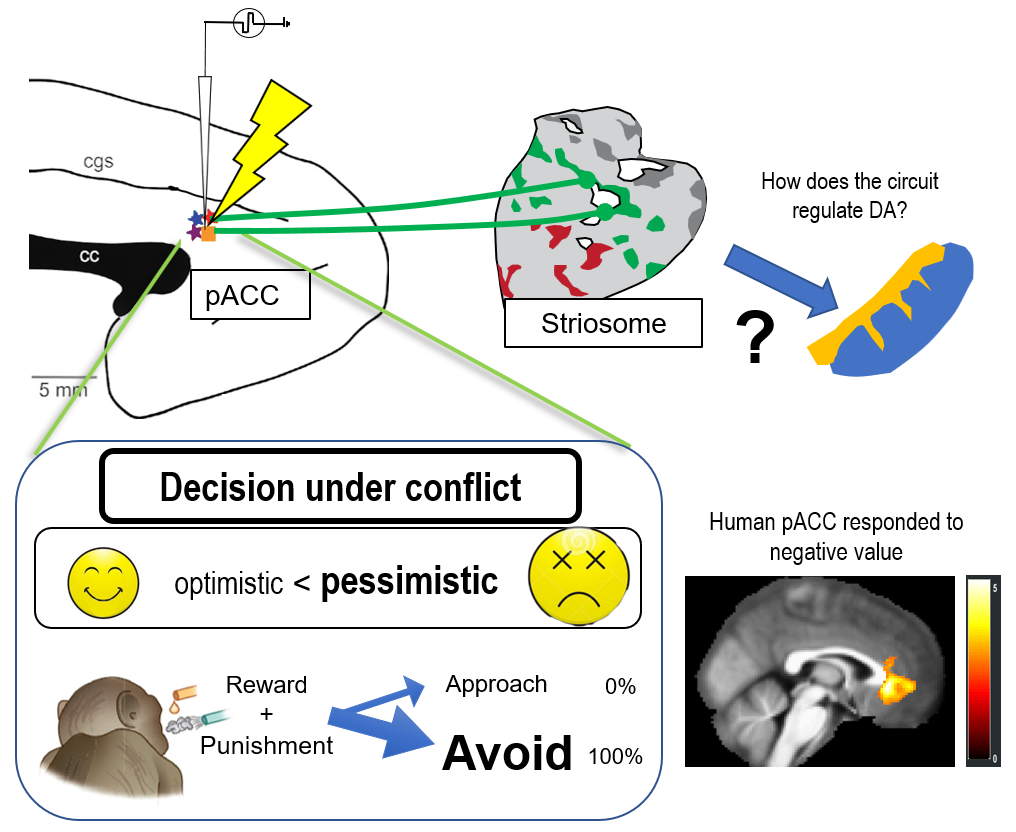
Fig 1: Anxiety-related circuitry in the limbic system. Microstimulation pf pACC induced pessimistic decision-making under conflict. We will analyze the mechanism of the anxiety-related circuitry by focusing on the cortico-basal ganglia circuitry in the NHPs.
Research Topic 1: Genetic approach to identify the primate anxiety-related circuits.
We focus on the cortico-basal ganglia circuit to understand how the limbic system induces anxiety and mood disorders. Our previous studies have shown that the downstream region of the limbic cortex in primates contains a striatal striosome compartment, raising the possibility that the anxiety-related circuitry could regulate the nigrostriatal dopamine cells. We will test this hypothesis in primates using genetic techniques to elucidate the mechanism of the generation of anxiety-like states. With the Ap-Av conflict tasks, we will newly introduce chemogenetics manipulation to search for the brain region responsible for emotionally abnormal decision-making (Figure 2a).
Research Topic 2: Physiological approach to elucidate the interareal communication of the primate limbic systems.
Primates have very large-scale brain network, consisting of massive interareal interactions, which could be acquired through evolution. In particular, the limbic systems of human and macaque monkeys contain specific cellular and network basis such as Von Economo Neuron and neuronal synchronization. We will combine microstimulation and fMRIs to identify the areas with long-distance communication. We will then perform multi-site simultaneous recording to reveal the interareal interaction among the regions in the limbic systems by focusing on their neural oscillations (Figure 2b).
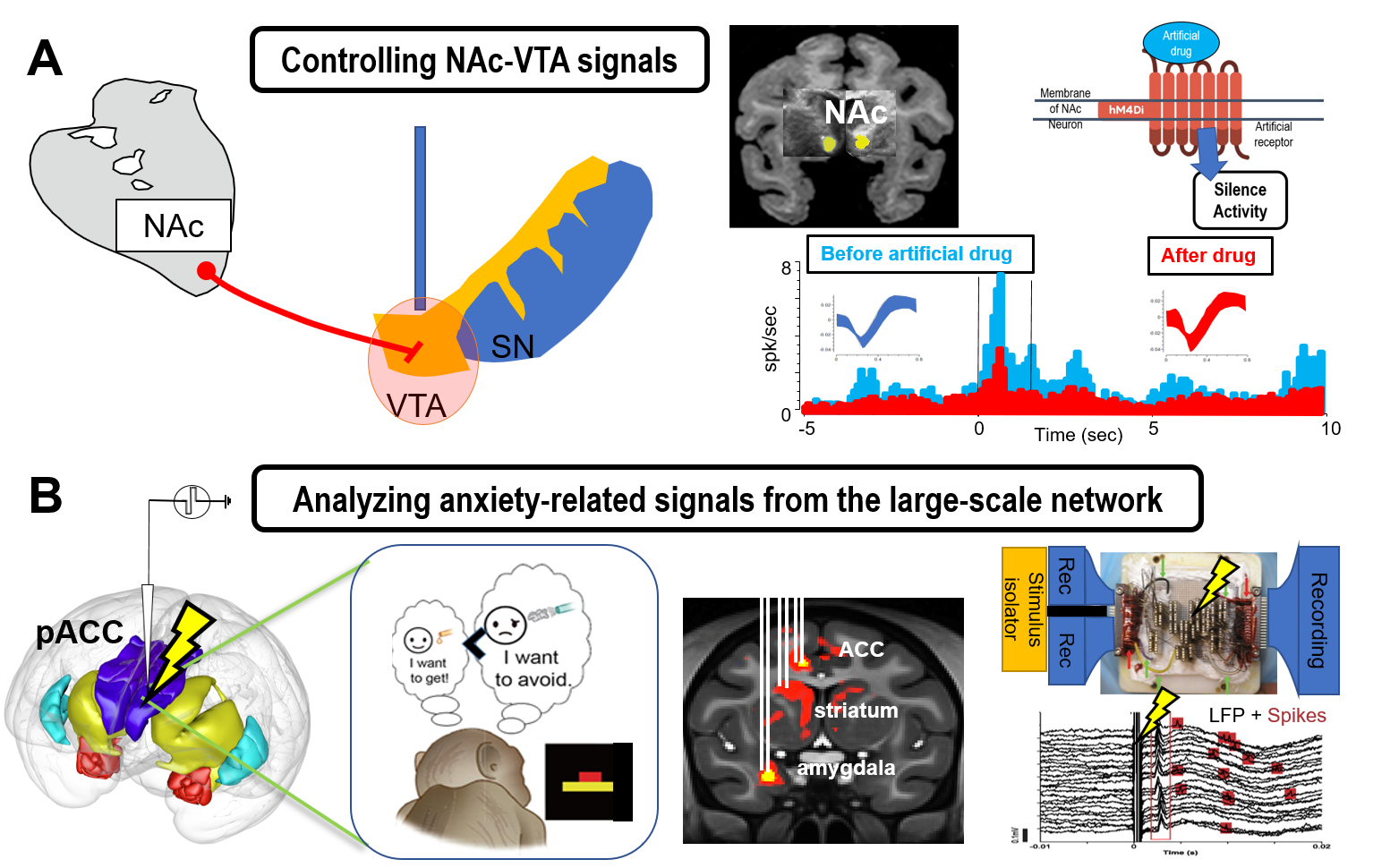
Fig 2: Genetic and physiological approaches to the primate anxiety network. A. Chemogenetics manipulation of the dopamine-controlling circuitry. B. Simultaneous recording of spikes and neural oscillations from the large-scale anxiety-related network in the primate limbic systems.
Members

Ken-ichi Amemori
- Position
- Associate Professor
- Laboratory Website
-
https://ashbi.kyoto-u.ac.jp/lab-sites/amemori/
-
Jungmin Oh
Program-Specific Researcher
Publications
Ironside, M+., Amemori, K+., McGrath, C. L., Pedersen, L. M., Kang, M. S., Amemori, S., Frank, M. J., Graybiel, A. M. and Pizzagalli, D. A. Approach-avoidance conflict in major depression: Congruent neural findings in human and non-human primates. Biological Psychiatry, 87 (2020): 399-408. (+: equal contribution)
Amemori, S., Amemori, K., Yoshida, T., Papageorgiou, G. K., Xu, R., Shimazu, H., Desimone, R. and Graybiel, A. M. Microstimulation of primate neocortex targeting striosomes induces negative decision-making. European Journal of Neuroscience, 51 (2020): 731-741.
Amemori, K., Amemori, S., Gibson, D. J., and Graybiel, A. M. Striatal beta oscillation and neuronal activity in the primate caudate nucleus differentially represent valence and arousal under approach-avoidance conflict. Frontiers in Neuroscience, 14 (2020): 89.
Amemori, K.+, Amemori, S.+, Gibson, D. J. and Graybiel, A. M. Striatal microstimulation induces persistent and repetitive negative decision-making predicted by striatal beta-band oscillation. Neuron, 99 (2018): 829-841. (+: equal contribution)
Friedman, A., Homma, D., Bloem, B., Gibb, L. G., Amemori, K., Hu, D., Delcasso, S., Truong, T. F., Yang, J., Hood, A. S., Mikofalvy, K. A., Beck, D. W., Nguyen, N., Nelson, E. D., Toro Arana, S. E., Vorder Bruegge, R. H., Goosens K. A. and Graybiel A. M. Chronic stress alters striosome-circuit dynamics, leading to aberrant decision-making. Cell, 171 (2017):1191-1205.
Desrocher, T. M., Amemori, K. and Graybiel, A. M. Habit learning by naive macaques is marked by dynamic response sharpening of striatal neurons representing the cost and outcome of acquired action sequences. Neuron, 87 (2015): 853-868.
Friedman, A., Homma, D., Gibb, L., Amemori, K., Rubin, S., Hood, A., Riad, M. and Graybiel, A. M. A corticostriatal path targeting striosomes controls decision-making under conflict. Cell, 161 (2015): 1320-1333.
Amemori, K., Amemori, S. and Graybiel, A. M. Motivation and affective judgments differentially recruit neurons in the primate dorsolateral prefrontal and anterior cingulate cortex. Journal of Neuroscience, 35 (2015): 1939-1953.
Amemori, K. and Graybiel, A. M. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nature Neuroscience, 15 (2012): 776–785.
Amemori, K.+, Gibb, L. G.+ and Graybiel, A. M.* Shifting responsibly: the importance of striatal modularity to reinforcement learning in uncertain environments. Frontiers in Human Neuroscience, 5 (2011): 47. (+: equal contribution)
