News
February 19, 2020
Cracking the code of egg production
Kyoto scientists discover master “on” switch for egg development in mice
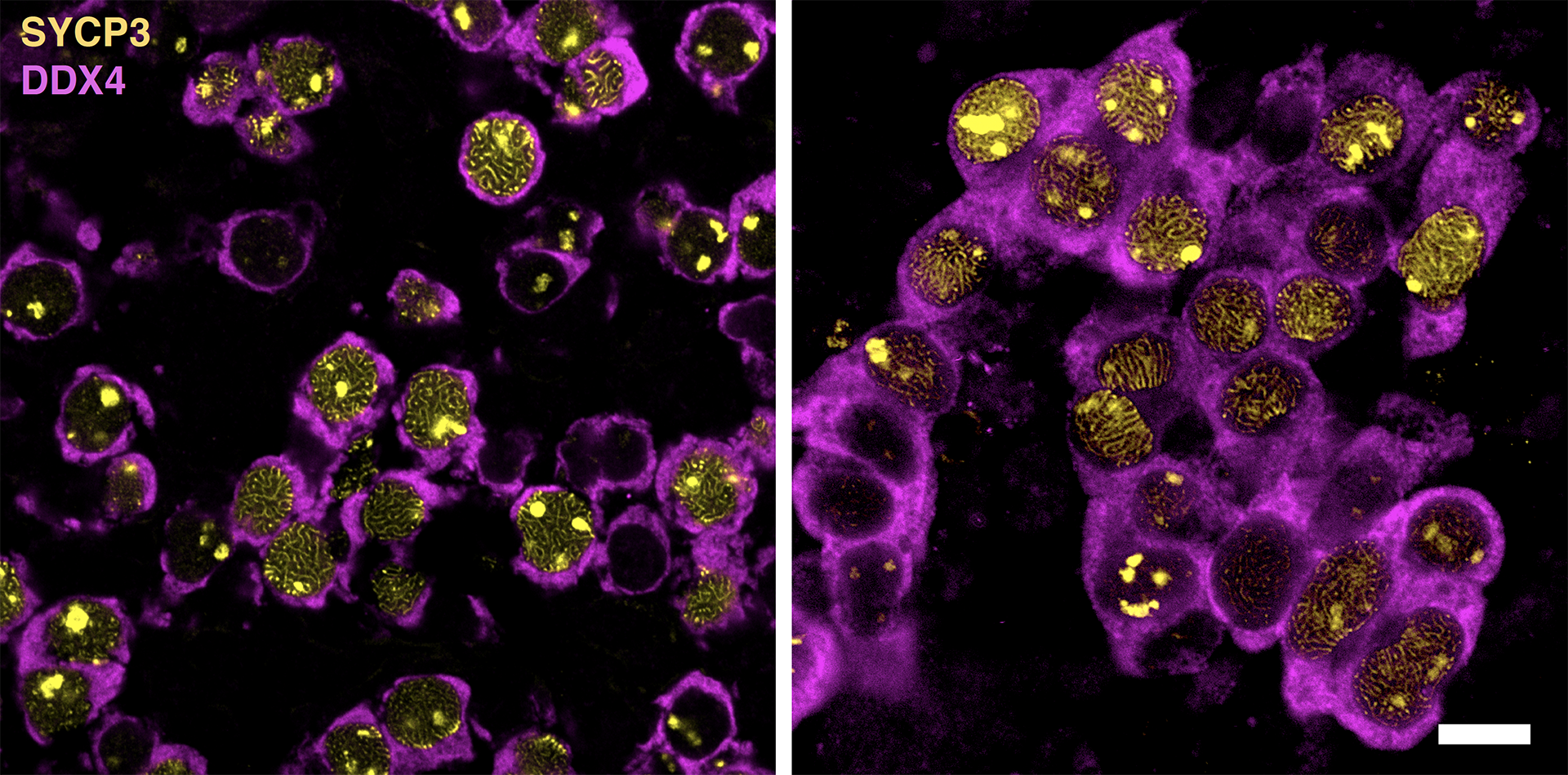
Left: E15.5 Oocytes, Right: mPGCLCs+ZGLP1, Scale bar=10μm
As mammalian embryos develop, the precursor sex cells (known as germ cells) become programmed to produce either eggs in females or sperm in males. However, the mechanism underlying how germ cells commit to following the correct differentiation path has intrigued scientists.
Past studies done in mice have suggested that retinoic acid is the chemical signal that launches the egg-making program in germ cells. But to date, it has been impossible to understand exactly how, because of the complex interactions between germ cells and surrounding non-germ cells in the embryonic sexual organs.
Now, writing in the journal Science, a group at the Institute for the Advanced Study of Human Biology (ASHBi), Kyoto University, has reported that retinoic acid plays only a helping role and that another molecule, bone morphogenetic protein (BMP), actually triggers egg production. Using their own technique for culturing mouse germ cells, the team demonstrated that BMP activates the egg-making process by turning on a single gene, named Zglp1.
“The sex of embryonic germ cells is not primarily decided by the sex chromosome make-up (XX or XY) of the germ cells themselves, but by signals from surrounding cells,” says Mitinori Saitou, leader of the Kyoto University team. “After we found that retinoic acid alone does not determine the sex of germ cells, we realized that we were looking at some much more complex mechanisms that may counter our current understanding.”
By studying germ cells derived from mouse stem cells and by using a controlled laboratory environment, the researchers were able to remove any interference that would have come from the surrounding cells present in embryonic sex organs. These cultured germ cells had the potential to become either eggs or sperm.
The team showed that adding BMP was enough to initiate egg development in the germ cells. Retinoic acid promoted the transformation process when added to germ cells with BMP but not when added on its own.
Analysis of gene expression patterns in germ cells before and after BMP addition revealed which genes had been switched on. The researchers identified eight activated genes that were potentially involved in sex determination. Experiments on germ cells lacking each of the eight genes, one by one, showed that only the gene Zglp1 was absolutely necessary for stimulating egg production.
Furthermore, artificially over-activating Zglp1 alone was enough to launch the egg-making program, without the need for BMP as a trigger. In turn, germ cells with an over-active Zglp1 gene showed decreased expression of 294 genes and increased expression of 489 genes, including some genes known to be involved in egg development. Addition of retinoic acid enhanced the expression of egg-development genes even more.
In support of the cell culture test results, egg production was prevented in female mice that were completely missing the Zglp1 gene. In male mice without the gene, germ cells still followed the sperm-making pathway but the adults were infertile, indicating that although Zglp1 is not necessary for sperm production, it is required for sperm maturation. Further animal experiments confirmed that the Zglp1 gene was getting switched on in females but not males at a time of embryo development consistent with the sex determination of germ cells.
BMP acts as a signal to switch on the Zglp1 gene thereby prompting egg production, whereas retinoic acid provides a signal that contributes to egg maturation, the authors conclude.
Saitou says, “It’s quite surprising to find that only a single gene, Zglp1, is sufficient for determining the fate of developing germ cells. I think we can also generalize these findings to human germ cells, which develop in a similar way. Our techniques and the insights that they provide will better equip researchers looking into human fertility and genetic diseases.”
Credit: Nano Pico Science
