R. Yamamoto Group
Stem Cell Biology & Hematology

Research Overview
Stem cells are characterized by two features, self-renewal activity and multipotency. Stem cells themselves gradually change with aging, causing several kinds of diseases and defects. However, the biology and mechanisms are to be determined. Our group focuses on blood stem cells (hematopoietic stem cells, HSCs) among several kinds of stem cells.
HSCs reside at the top of the hierarchy of the hematopoietic system. The aging and dysfunction of this long-lived adult stem cell populations are thought to underlie the hematopoietic system decline and dysfunction associated with physiological aging or diseases. Age-related dysfunction of the hematopoietic system includes reduced lymphopoiesis and excessive myeloid production. The acquisition of specific somatic mutations in HSCs results in clonal hematopoiesis with aging, which is a clonally expanded blood system originating from the mutated HSCs. The genomic landscape in clonal hematopoiesis and myeloid malignancies provided insights into the evolution of hematopoietic malignancies from clonal hematopoiesis with aging. However, the mechanisms underlying clonal hematopoiesis and myeloid malignancies remains to be assessed. A better understanding of these mechanisms is critical to promote healthy aging.
Our goals
- Elucidate the HSC biology and mechanisms underlying self-renewal and multipotency of HSCs using mouse, non-human primate and human models
- Elucidate the biology and mechanisms of HSC aging and clonal hematopoiesis using a mouse, non-human primate and human models
- Elucidate the pathogenesis of acute myeloid leukemia (AML), myelodysplasia syndrome (MDS) and lymphoma using a mouse, non-human primate and human models
Projects
- Single-cell analysis of HSCs (stem cell transplantation, single-cell RNA-sequence, single-cell ATAC sequence, etc) to determine heterogeneity, HSC mechanisms, and HSC aging
- Establish a gene-modified mouse and non-human primate model
- Apply new techniques such as mathematics to analysis of 1. and 2.
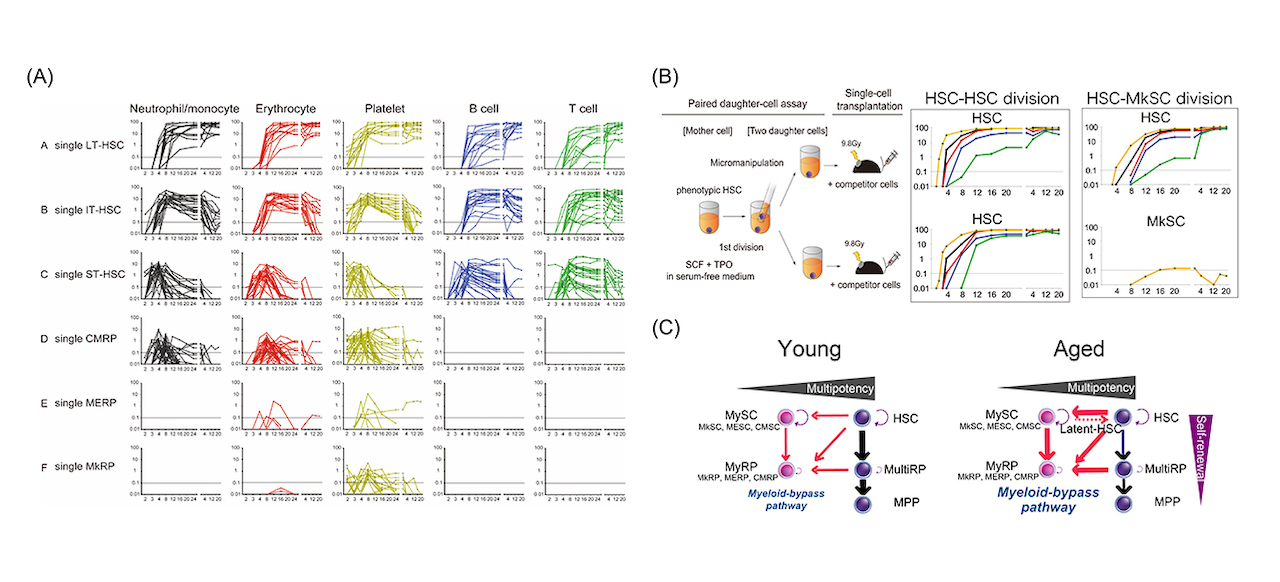
Fig1: (A) Chimerism dynamics after single cell transplantation (B) Paired-daughter cell assay to determine cell division pattern of single HSCs (C) Revised hematopoiesis model via myeloid-bypass pathway.
Fig2: Overview of our lab
Members

Ryo Yamamoto
- Position
- Associate Professor
- Laboratory Website
-
https://ashbi.kyoto-u.ac.jp/lab-sites/ryo-yamamoto/
-
Jun Ooehara
Specially Contracted Staff Member
-
Toshiko Sato
Part Time Researcher
-
Machiko Yoshioka
Assistant Technical Staff
-
Natsuki Hosho
Dispatched Employee
-
Keimyo Imamura
Graduate Student
-
Yuuta Suzuki
Graduate Student
-
Youngeun Been
Graduate Student
Publications
Wilkinson, A. C.*, R. Ishida*, M. Kikuchi, K. Sudo, M. Morita, R. V. Crisostomo, R. Yamamoto, K. M. Loh, Y. Nakamura, M. Watanabe, H. Nakauchi, and S. Yamazaki. “Long-term ex vivo expansion of hematopoietic stem cells affords non-conditioned transplantation.” Nature, 571(2019): 117-121. (*Equal contribution)
Yamamoto, R.*, A. C. Wilkinson*, and H. Nakauchi. “Changing concepts in hematopoietic stem cells.”Science, 362 (2018): 895-96.(*Equal contribution)
Yamamoto, R., A. C. Wilkinson, J. Ooehara, X. Lan, C. Y. Lai, Y. Nakauchi, J. K. Pritchard, and H. Nakauchi. “Large-Scale Clonal Analysis Resolves Aging of the Mouse Hematopoietic Stem Cell Compartment.” Cell Stem Cell, 22 (2018): 600-07 e4.
Ghosn, E. E., J. Waters, M. Phillips, R. Yamamoto, B. R. Long, Y. Yang, R. Gerstein, C. A. Stoddart, H. Nakauchi, and L. A. Herzenberg. “Fetal Hematopoietic Stem Cell Transplantation Fails to Fully Regenerate the B-Lymphocyte Compartment.” Stem Cell Reports, 6 (2016): 137-49.
Matsunawa, M.*, R. Yamamoto*, M. Sanada*, A. Sato-Otsubo, Y. Shiozawa, K. Yoshida, M. Otsu, Y. Shiraishi, S. Miyano, K. Isono, H. Koseki, H. Nakauchi, and S. Ogawa. “Haploinsufficiency of Sf3b1 Leads to Compromised Stem Cell Function but Not to Myelodysplasia.” Leukemia, 28 (2014): 1844-50.(*Equal contribution)
Yamamoto, R.*, Y. Morita*, J. Ooehara, S. Hamanaka, M. Onodera, K. L. Rudolph, H. Ema, and H. Nakauchi. “Clonal Analysis Unveils Self-Renewing Lineage-Restricted Progenitors Generated Directly from Hematopoietic Stem Cells.” Cell, 154 (2013): 1112-26.(*Equal contribution)
Ghosn, E. E., R. Yamamoto, S. Hamanaka, Y. Yang, L. A. Herzenberg, H. Nakauchi, and L. A. Herzenberg. “Distinct B-Cell Lineage Commitment Distinguishes Adult Bone Marrow Hematopoietic Stem Cells.” Proc Natl Acad Sci USA, 109 (2012): 5394-8.
Yoshida, K*., M. Sanada*, Y. Shiraishi*, D. Nowak*, Y. Nagata*, R. Yamamoto, Y. Sato, A. Sato-Otsubo, A. Kon, M. Nagasaki, G. Chalkidis, Y. Suzuki, M. Shiosaka, R. Kawahata, T. Yamaguchi, M. Otsu, N. Obara, M. Sakata-Yanagimoto, K. Ishiyama, H. Mori, F. Nolte, W. K. Hofmann, S. Miyawaki, S. Sugano, C. Haferlach, H. P. Koeffler, L. Y. Shih, T. Haferlach, S. Chiba, H. Nakauchi, S. Miyano, and S. Ogawa. “Frequent Pathway Mutations of Splicing Machinery in Myelodysplasia.” Nature, 478 (2011): 64-9.(*Equal contribution)
Yamamoto, R., M. Nishikori, M. Tashima, T. Sakai, T. Ichinohe, A. Takaori-Kondo, K. Ohmori, and T. Uchiyama. “B7-H1 Expression Is Regulated by Mek/Erk Signaling Pathway in Anaplastic Large Cell Lymphoma and Hodgkin Lymphoma.” Cancer Sci, 100 (2009): 2093-100.
Yamamoto, R., M. Nishikori, T. Kitawaki, T. Sakai, M. Hishizawa, M. Tashima, T. Kondo, K. Ohmori, M. Kurata, T. Hayashi, and T. Uchiyama. “PD-1-PD-1 Ligand Interaction Contributes to Immunosuppressive Microenvironment of Hodgkin Lymphoma.” Blood, 111 (2008): 3220-4.
