News
January 5, 2024
Lighting the Circuits to Risky Decision-Making
Researchers at Kyoto University identify and selectively manipulate the neural circuits underlying the balance of risk vs. reward decision-making using optogenetics in primates.
KYOTO, Japan – Jan. 5th 2024
Schematic Diagram representing the Concept of Risk vs Reward Decision-Making.
Bananas represent the reward and the crocodile represents the risk. The blue path is a low risk-low return decision, while the pink path is a high risk-high return decision.
Credit:Trais
Life consists of infinite possibilities — appearing in the real world as multiple choices, that then require decision-making in order to determine the best course of action. However, with every choice there also exists a certain amount of uncertainty or ‘risk’. Therefore, behind every decision, lies an intricate evaluation process that balances the ‘risks’ and ‘rewards’ associated with taking such actions. This can, in extreme cases, manifest itself as a pathological behavioral state of high risk-high return (HH) and low risk-low return (LL) decision processing that has been associated with gambling disorders.
Although these higher cognitive processes occur seamlessly within the cerebral cortex of our brains — dozens to hundreds of times daily — the exact underlying neural circuits have remained elusive due to the technical difficulties of specifically targeting and manipulating these neural circuits.
A new study published Science, from a team of researchers led by Dr. Tadashi Isa at the Institute for the Advanced Study of Human Biology (WPI-ASHBi) and Graduate School of Medicine/Kyoto University, have identified and selectively manipulated using optogenetics — a method that can modulate the activity of specific neurons with light — the distinct neural circuits responsible for balancing risk vs. reward-return decision-making in primates. They show the behavioral changes resulting from stimulating these circuits accumulate over time and have long-term consequences — independent of any stimulus — providing insights into potential mechanisms underlying pathological risk-taking behaviors such as gambling disorders.
Various experimental paradigms have been developed to evaluate decision-making behavior, with the Iowa Gambling Task being arguably the most famous. However, such neuropsychological tasks are often limited by their design, as they cannot sufficiently uncouple higher-order cognitive processes.
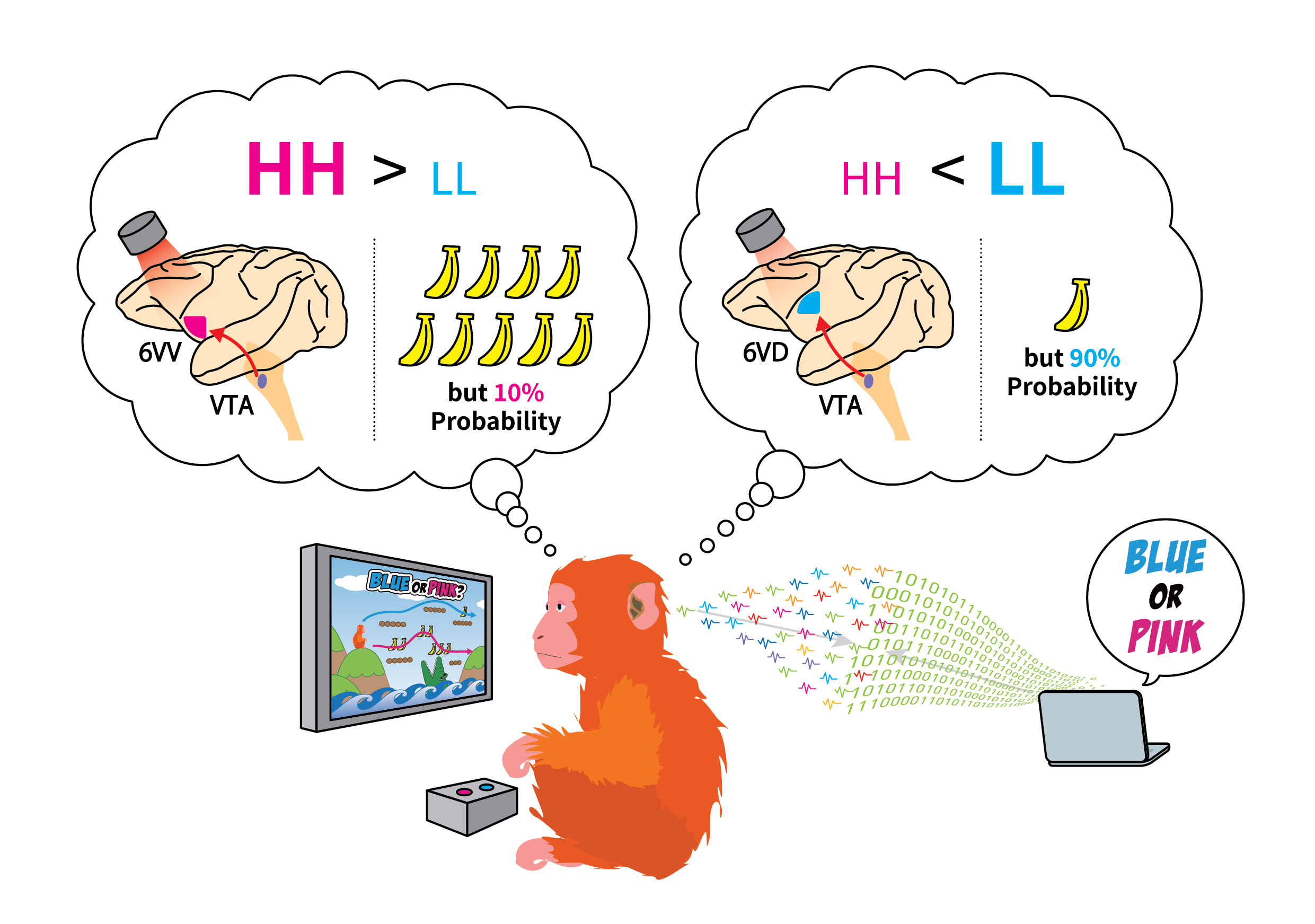
To determine the ‘pure’ choice bias between HH and LL decisions, Isa and colleagues first designed their own decision paradigm to uncouple risk-dependent choice behavior from other higher-order cognitive processes. Using eye movement to indicate their choice, macaque monkeys were trained to perform a cue/target choice task with water as their reward, consisting of 5 different HH-LL choices across 5 different sets of equivalent ‘expected value’ (volume of reward awarded multiplied by probability), making a total of 25 potential options. Consistent with other primate studies that looked at risk-behavior, the authors found that primates had an inherent bias for HH over LL choices.
In the early 20th century, the cerebral cortex was mapped into 52 regions, known as Brodmann areas, based on their distinct cellular morphology and organization. The deeper, or ventral, parts of Brodmann area 6 (area 6V), were long thought to only function as a motor area in humans and primates. But more recently, regions overlapping area 6V have also been associated with decision-making processes, though direct evidence supporting such a function has been lacking.
By pharmacologically inactivating several candidate frontal brain regions, using the selective GABAA receptor agonist muscimol, the authors found that the ventral part of area 6V (area 6VV) to be responsible for the HH choice behavior. Interestingly, despite the orbitofrontal cortex (OFC) and the dorsal anterior cingulate cortex (aACC) being considered to play central roles in reward-based decision-making in monkeys, inactivating these regions had little effect on preference for HH choice.
“Indeed, we were really surprised that neither the OFC nor the aACC were important for risk-dependent decision-making” comments Dr. Ryo Sasaki, the first author of the study.
The ventral tegmental area (VTA) of the brain is essential for reward-associated processes, which is integral to risk-related decision-making. A subpopulation of dopaminergic neurons residing in the VTA are connected to the prefrontal cortex, including area 6V, also known as the mesofrontal (or the mesocortical) pathway.
To dissect the specific role of the mesofrontal pathway in risk-dependent decision-making, Isa and collaborators used an elegant optogenetic strategy, whereby an array consisting of 29 LED lights coupled to electrocorticogram (ECoG) electrodes was engineered (dimensions: 19mm x 12mm) and implanted into area 6V of primate brains expressing photoactivatable proteins in VTA neurons. During the narrow time window of decision-making, the authors precisely manipulated the neural activity of defined VTA terminals in area 6V by turning ON specific LEDs in their array, while simultaneously recording the activity within area 6V, that also included more superficial, or dorsal regions (area 6VD; approximately 2-3mm above area 6VV). The authors uncovered two subcircuits within the mesofrontal pathway with distinct roles in risk-dependent decision-making. They found HH-preference was dependent on the VTA-6VV pathway, whereas LL preference was dependent on the VTA-6VD pathway.
“The spatiotemporal resolution of our LED/ECoG array was essential in distinguishing the VTA-6VV and VTA-6VD pathways and deciphering their distinct functional roles in risk-dependent decision-making” claims Sasaki.
These findings were further validated by computational decoding, which recapitulated the choice preference behavior induced by photostimulation in primates in silico.
Interestingly, upon repetitive stimulation of either the VTA-6VV or the VTA-6VD pathways, Isa and coauthors observed cumulative effects that persisted over time, leading to long-term changes in preference for HH and LL choice in primates, respectively — independent of any photostimulation. Isa comments, “…such long-term changes in choice behavior were rather unexpected” and he adds, “… but this may now also offer a mechanistic explanation for how gambling disorders arise”.
Exactly how these distinct circuits contribute to balancing our day-to-day decision-making remains unclear, but the authors believe other brain regions are likely to also contribute to this process.
“Considering the similarities (in structure and function) between human and non-human primate brains, our findings may have potential therapeutic implications, and even applications in the future, for the treatment of pathological forms of risk-taking such as gambling disorders”, he says.
These findings were published in Science on January 5th 2024.
By Spyros Goulas, PhD
Scientific Advisor
Paper information:
Sasaki, R., Ohta, Y., Onoe, H., Yamaguchi, R., Miyamoto, T., Tokuda, T., Tamaki, Y., Isa, K., Takahashi, J., Kobayashi, K., Ohta, J., & Isa, T. (2024). Balancing Risk-Return Decisions by Manipulating the Mesofrontal Circuits in Primates. Science. https://doi.org/10.1126/science.adj6645
