Ueno Group
Immunology
Research Overview
CD4+ T cells represent a major cell component of the adaptive immune system. Naive CD4+ T cells differentiate into different types of effectors in periphery upon encounter with antigen-presenting dendritic cells. Effectors are composed of fairly diverse cell populations called subsets. During the initial differentiation process, signals via various receptors on T cells, e.g., T cell receptor, co-stimulatory molecules, and cytokine receptors, affect the differentiation fate. While the function of CD4+ T cell subsets is relatively conserved among mammals, the major pathways involved in the differentiation process are different in many instances. Furthermore, even within human-derived naïve CD4+ T cells, cells isolated from different human organs, e.g., cord blood, adult peripheral blood, tonsils, and spleen; and isolated from subjects with different ages, e.g., infants, young adults, and elderly, display different features and differentiation trajectory. The molecular and epigenetic mechanisms underlying the differences in the CD4+ T cell differentiation among species, anatomical sites, and ages remain ill defined. In this project, we aim to define the genetic, transcriptomic, and epigenetic factors that regulate the differentiation of naïve CD4+ T cells that differ in origins. In parallel, we will analyze monocytes as a representative cell type within the innate immune system. We anticipate to reveal the molecular mechanism by which immune cells respond differently among species; mouse, macaques, and humans (Aim1); among anatomical sites in human organs; peripheral blood, tonsils, spleen, and gut (Aim 2); among different ages (Aim 3).
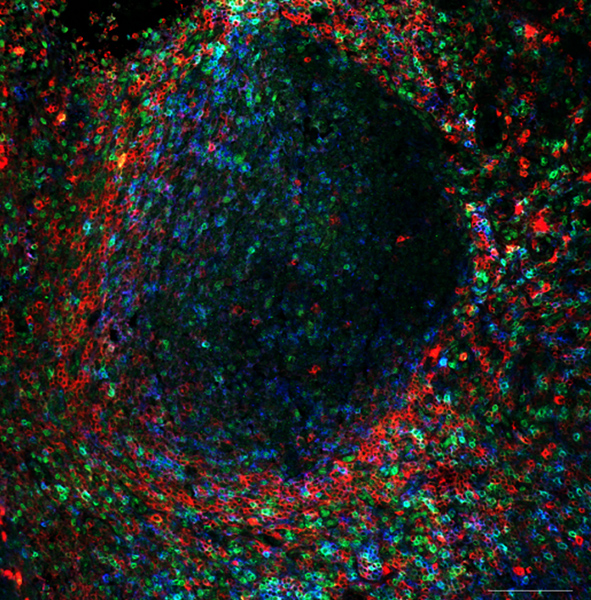
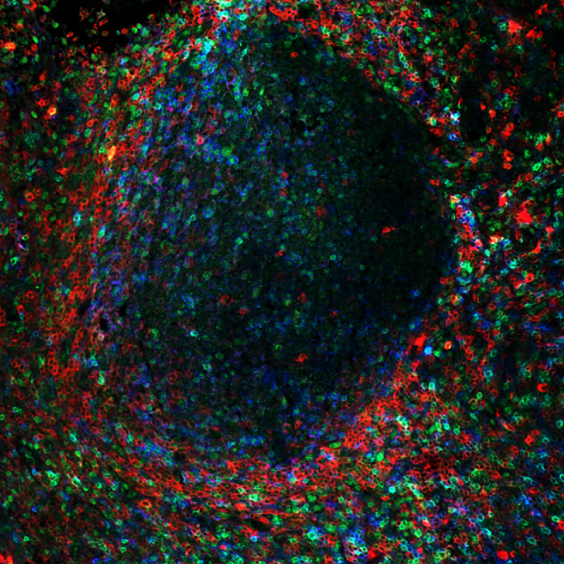
Figure 1: CD4+ T cell subsets within germinal centers in human tonsils.
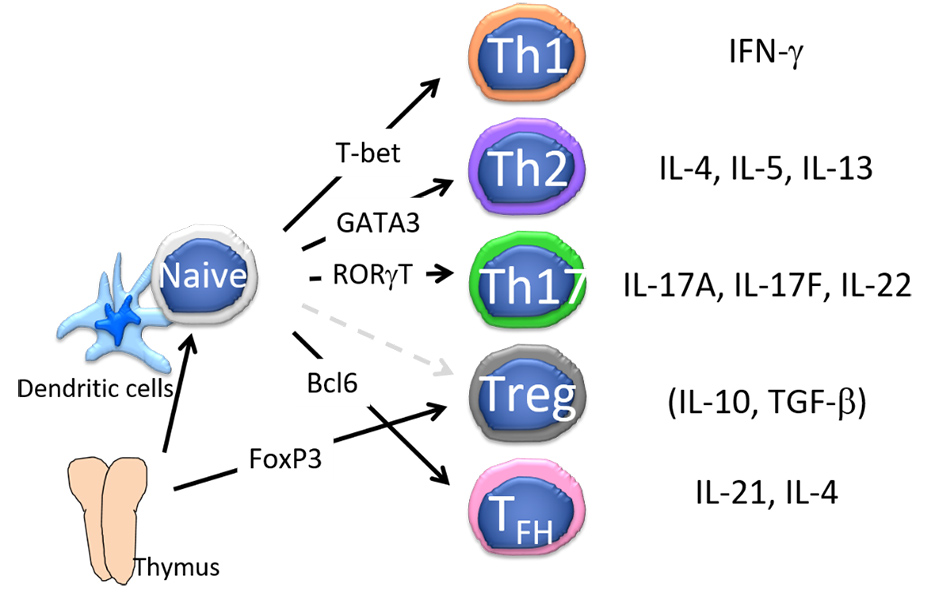
Figure 2: CD4+ T cell subsets. The differentiation of naïve CD4+ T cells into effector subsets depends on a specific transcription factor. The major pathways that induces the specific transcription factors differ among species.
Members

Hideki Ueno
- Position
- Professor
- Laboratory Website
-
https://immunol.med.kyoto-u.ac.jp/en/
-
Hiroyuki Yoshitomi
Associate Professor
-
Mio Hamatani
Assistant Professor
-
Yoshitaka Honda
Assistant Professor
-
Shuhe Ma
Program-Specific Researcher
-
Xinxin Xue
RA
-
Dongyun Lu
RA
Publications
Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108-21.
Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, Xu H, Pascual V, Banchereau J, Garcia-Sastre A, Palucka AK, Ramilo O*, Ueno H*. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5(176):176ra32. PMCID: 3621097. * Equal contributors.
Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15(9):856-65.
Jacquemin C, Schmitt N, Contin-Bordes C, Liu Y, Narayanan P, Seneschal J, Maurouard T, Dougall D, Davizon ES, Dumortier H, Douchet I, Raffray L, Richez C, Lazaro E, Duffau P, Truchetet ME, Khoryati L, Mercie P, Couzi L, Merville P, Schaeverbeke T, Viallard JF, Pellegrin JL, Moreau JF, Muller S, Zurawski S, Coffman RL, Pascual V, Ueno H*, Blanco P*. OX40 Ligand Contributes to Human Lupus Pathogenesis by Promoting T Follicular Helper Response. Immunity. 2015;42(6):1159-70. * Equal contributors.
Schmitt N, Liu Y, Bentebibel SE, Ueno H. Molecular Mechanisms Regulating T Helper 1 versus T Follicular Helper Cell Differentiation in Humans. Cell Rep. 2016;16(4):1082-95.
