News
August 30, 2023
Cell interactions in abnormal structures in aged kidneys: Potential therapeutic target for chronic kidney disease
Credits: Hiroko Uchida
Chronic kidney disease is a condition in which kidney function declines, causing significant health issues in patients. A previous study led by Dr. Motoko Yanagita found that tertiary lymphoid tissues (TLTs), which develop in several kidney diseases, inhibit the repair of kidney injury and are associated with poorer prognosis connected to the development of chronic kidney disease. A new study investigated the role of TLTs in exacerbating kidney injury and the study’s findings were published in the Journal of the American Society of Nephrology.
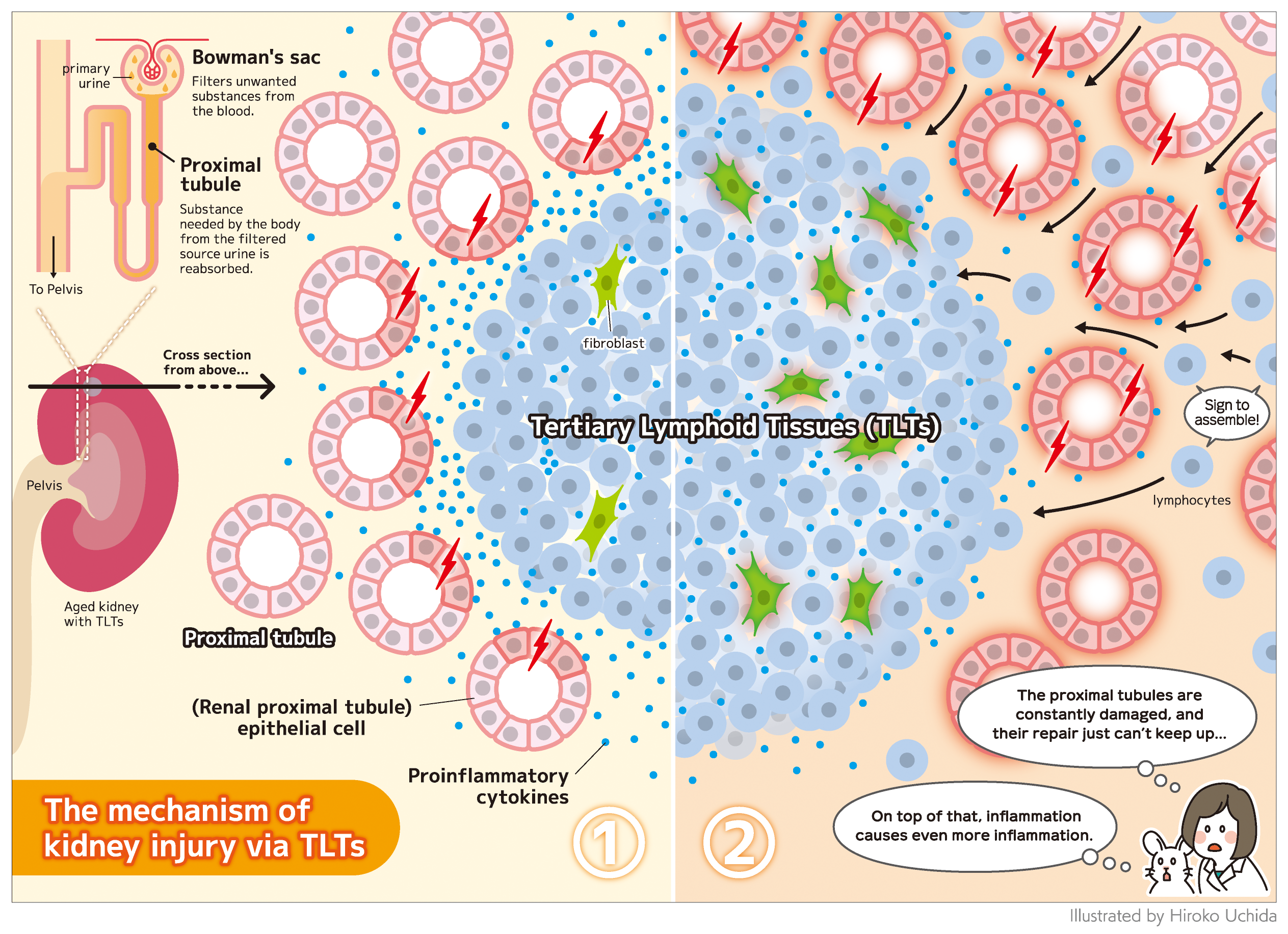
TLTs are abnormal structures composed of lymphoid tissue, including lymphocytes called T and B cells, as well as functionally specialized fibroblasts, a type of cell usually involved in forming connective tissues. TLTs are also present in injured fibrotic kidneys. However, the mechanism of TLT expansion and its effect on renal regeneration remain unclear.
To elucidate these issues, the Kyoto-based research team used single-nucleus RNA-sequencing (snRNA-seq) to investigate the interaction between renal parenchymal cells (cells constituting the bulk of functional material in the kidney) and immune cells in the kidneys of older mice. After ischemia–reperfusion injury was induced in the kidney on one side in experimental mice, the kidney was harvested from both the experimental mice and control mice with no injury. Subsequently, snRNA-seq was performed on the nuclei of cells extracted from the kidneys in order to determine the expression of ligands—molecules that these cells use to interact with one another. In addition, renal biopsy specimens from two kidney transplant recipients were also examined, and the expression of molecules focused on the mice study was assessed.
Histological examination of the injured mice kidneys revealed extensive atrophy of proximal tubular cells, interstitial fibrosis, and the presence of multiple TLTs in the renal cortex.
The researchers identified proinflammatory proximal tubular cells and fibroblasts among the renal parenchymal cells in aged-injured kidneys with TLTs. Interactions between these proinflammatory renal parenchymal cells and immune cells within and outside TLTs have the potential to promote inflammation, TLT expansion, and kidney injury. Proinflammatory proximal tubular cells that may recruit and activate immune cells via several types of proinflammatory cytokine production are preferentially localized around TLTs. TLTs are suggested to directly injure the proximal tubules and promote their proinflammatory property via producing cytokines such as interferon gamma and tumor necrosis factor alpha. Proinflammatory fibroblasts within TLTs may also contribute to the recruitment, retention, survival, and proliferation of lymphocytes via cytokine or chemokine production. In addition, they found that some transcription factors induced by interferon were highly activated in proinflammatory fibroblasts within TLTs in mouse and human kidneys. These results and in vitro experiments suggest that interferon gamma may promote the proinflammatory phenotype of fibroblasts within TLTs. The proinflammatory proximal tubules and proinflammatory fibroblasts were also identified in human transplanted kidneys with TLTs.
These findings may lead to the development of new therapeutic strategies. “Drugs that inhibit these cytokines may have the potential to prevent progressive injury and TLT expansion in aged-injured kidneys,” said Dr. Takahisa Yoshikawa, the study’s lead author. “However, further studies are needed to confirm the effect of these drugs.”
ThinkSCIENCE, Inc. (Tokyo, Japan)
Paper Information
Yoshikawa, T., Oguchi, A., Toriu, N., Sato, Y., Kobayashi, T., Ogawa, O., Haga, H., Sakurai, S., Yamamoto, T., Murakawa, Y. and Yanagita, Y. (2023) Tertiary lymphoid tissues are microenvironments with intensive interactions between immune cells and proinflammatory parenchymal cells in aged kidneys. Journal of the American Society of Nephrology, DOI: 10.1681/ASN.0000000000000202
Grant Information
This research was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Numbers AMED-CREST 22ek0310020, 22gm1210009, 22zf0127003, 21gm5010002, and 21lm0203006; and the Japan Society for the Promotion of Science under a KAKENHI Grant-in-Aid for Scientific Research B (20H03697).
