News
March 9, 2023
Questionable practices identified by an examination of therapeutic plan reviews performed by certified committees under the Act on the Safety of Regenerative Medicine
A research team led by Dr. Tsunakuni Ikka of the National Cancer Center Japan and Dr. Misao Fujita of Kyoto University highlights the independence, integrity, and quality of reviews of therapeutic plans for regenerative medicine.
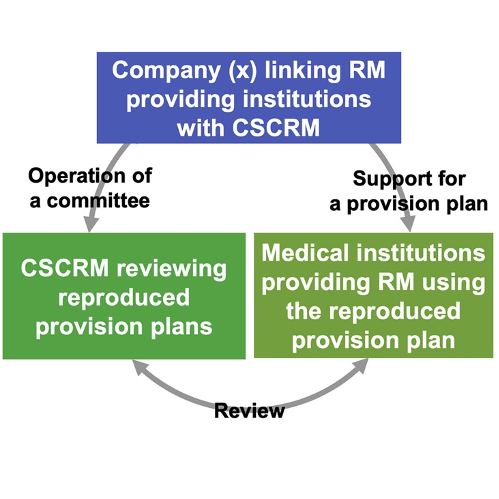
The Act on the Safety of Regenerative Medicine (ASRM) was enacted in 2013 to ensure that regenerative medicine is accessible to the public in Japan in a safe and timely manner. Under the ASRM, before implementing regenerative medicine therapy or research, medical institutions must submit their plans to a certified committee approved by the Minister of Health, Labour and Welfare for review. In this study, the team carefully evaluate the independence, integrity and quality of review committees and indicated problems with the current review process.
The researchers hope that these findings will generate social interest in the ongoing debate on the revision of the ASRM to ensure appropriate changes to the law to increase accountability and credibility to the operation of the review system and, help patients understand more about regenerative medicine and make better treatment choices.
The results of this study were published online in Stem Cell Reports on Feb 23, 2023.
Paper Information
Ikka, T*., Fujita, M*., Hatta, T., Isobe, T., Konomi, K., Onishi, T., Sanada, S., Sato, Y., Tashiro, S. and Tobita, M., (2023). Difficulties in ensuring review quality performed by committees under the Act on the Safety of Regenerative Medicine in Japan. Stem Cell Reports, DOI: 10.1016/j.stemcr.2023.01.013
