News
November 19, 2021
Gene regulation from the X chromosome during monkey development
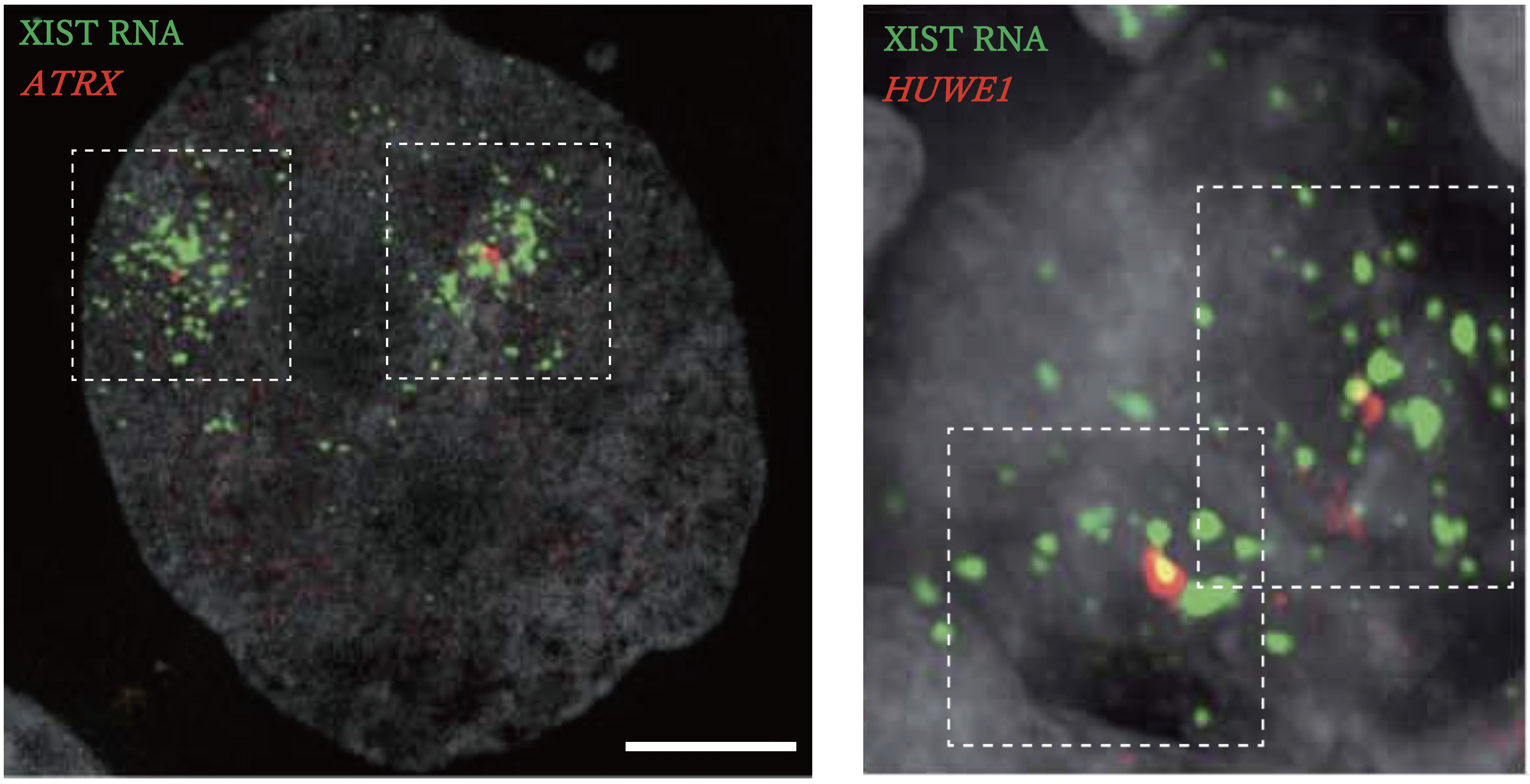
Left: Three-dimensional structured illumination microscopy (3D-SIM) images of XIST RNA (green) and X-linked genes (ATRX) (red) in a trophectoderm of female E8 embryos.
Right: 3D-SIM image of XIST RNA (green), X-linked genes (HUWE1) (red) in E57 female germ cells.
As an embryo grows into a whole human being, a number of key genetic events must occur. One of the two X chromosomes in the cells of females becomes inactive during early embryonic development. Because the study of human embryo development is heavily restricted for ethical reasons, scientists have turned to observing other primate mammals in order to understand this critical phenomenon in humans. A new study led by ASHBi researchers provides a detailed description of the dynamics of X-chromosome dosage compensation in monkeys, providing the most accurate understanding of X chromosome dosage compensation dynamics in humans to date.
Among the 46 chromosomes in human cells, females have two X chromosomes, one from each parent, and males have one X chromosome from the mother and one Y chromosome from the father. This pattern holds true for all mammals. Although both are sex chromosomes, they are very different: the X chromosome encodes more than 800 genes, whereas the Y chromosome encodes a relatively paltry number of around 50 genes.
The two X chromosomes in female embryos mean cells have two pairs of all the genes encoded on the X chromosome. However, expressing the genes on both chromosomes during development in an untimely manner can lead to disease, disability, and death. Therefore, one of the two X chromosomes is inactivated to achieve dosage compensation during the early development.
“X chromosome inactivation is regulated by XIST, a type of RNA that causes the chromosome to condense. The X chromosome inactivation and reactivation are critical for embryonic development,” explained ASHBi Director Mitinori Saitou, one of the lead authors of the study.
Overall, scientists have an elaborate understanding of X chromosome dosage compensation in mouse embryos. But mouse embryos and human embryos develop very differently, which is why Saitou and his colleagues have turned to studying monkey embryos, which make a far better model for predicting the events that happen in humans.
Indeed, past research from collaborations between Saitou and his ASHBi colleague, Dr. Ikuhiro Okamoto, another author of the study, has shown that the development of monkey and human embryos are quite similar from one another and quite distinct from the development of mouse embryos.
“We have found that monkey embryos are more suitable for studying human development than mouse embryos,” said Okamoto.
In fact, in the preimplantation embryos and placenta in mice, X chromosome inactivation only occurs on the paternal X chromosome, but either X chromosome can be inactivated in monkey and human embryos.
The new study examined how XIST interacts dynamically with the X chromosomes in developing female and male monkey embryos to regulate inactivation and reactivation.
In general, for X chromosome inactivation, XIST RNA wraps around the chromosome to condense it. This wrapping triggers a series of epigenetic events that eventually leads to the inactivation.
The study found that in embryos prior to implantation, both X chromosomes in the vast majority of female embryo cells, but not all, were wrapped by XIST RNA but still active. The same was true for the one X chromosome in the majority cells of males.
“We found that the chromosome would become compact but still remain active. This suggests that XIST RNA and the epigenetic modifications that follow are not sufficient for X chromosome inactivation in monkey,” said Saitou.
Instead, the X chromosome inactivation began in the cells of female embryos that were early post-implantation stage. The existence of an embryo does not necessarily mean pregnancy. Pregnancy happens only when the embryo has implanted itself into the uterine wall, an embryonic stage scientists term post-implantation. The study found that X chromosome inactivation in early post-implantation embryos only occurred in the one X chromosome wrapped by XIST RNA; the other became free of this RNA and was active.
“Our data suggest X chromosome inactivation happens rapidly after implantation but with remarkable cell heterogeneity,” remarked Saitou.
Understanding the dynamics of X chromosome inactivation during monkey embryonic development, stresses Saitou, provides a blueprint for understanding the early events of human embryonic development and may provide insight on why some embryos fail to survive, while others are eventually born.
“The regulation of X chromosome dosage compensation is distinct in mouse and monkey. This is critical, because we know monkey cells make a better model for the study of humans,” he said.
Paper Information
Ikuhiro Okamoto, Tomonori Nakamura, Kotaro Sasaki, Yukihiro Yabuta, Chizuru Iwatani, Hideaki Tsuchiya, Shin-ichiro Nakamura, Masatsugu Ema, Takuya Yamamoto, Mitinori Saitou, “The X-chromosome dosage compensation program during the development of cynomolgus monkeys”(2021), Science, DOI:https://doi.org/10.1126/science.abd8887
