
Chih-Yang Chen
Researcher (Isa-G)
- Position
- Assistant Professor
- Research Field
- Primate Neurophysiology
Research Overview
Cortical and subcortical interaction in saccade generation
One very unique feature of us primates is that we have foveated eyes. The primate retinas consist of densely packed photoreceptors at the foveal region but relatively loose in the periphery. In order to obtain the best vision with our eyes, we constantly move them to put the things we want to see onto our fovea. Such eye movements are usually ballistic, called saccades. In order to make a successful saccade requires multiple steps including making decision of the saccade target, preparing for the proper saccade and suppressing unwanted saccade, and also shift of attention to the saccade target. Because of the complex and dedicated nature of saccadic eye movements, it has been shown for decades that the cortical and subcortical brain areas controlling saccadic eye movements are also involved in cognitive function, such as attention and decision making. Several cortical areas, like frontal eye field (FEF) and lateral intraparietal area (LIP), and the subcortical area, superior colliculus (SC) had been shown to contribute to saccadic eye movement. However, whether each brain area serve specific function in specific time point in saccade generation or this is a joint work from multiple brain areas simultaneously is still not clear.
I have been investigating saccadic eye movements and the consequence of such movement altering our attention and visual perception during my Ph.D. research. I have found that there was correlation between the preparatory neuronal signal for saccades in the SC and the alternation of attention. I would like to further investigate the interaction in the saccade network using advanced calcium imaging and electrophysiological methods to simultaneously record multiple cortical and subcortical brain areas involving in saccade generation. To tease apart the role of each brain region, I would use the stat-of-the-art optogenetics tools to excite or inhibit these areas in various time point during saccade preparation and execution. To explain the above idea in more detail, I will train one small new world monkey, the marmosets, for saccadic tasks, such as gap task, delayed saccade, and memory-guided saccade task (Fig 1). After the marmosets are successfully trained, I will monitor the activity of neurons by performing simultaneous calcium signal recording in the brain areas involved in saccade generation while they are engaged in the saccade tasks (Fig 2a, 2b). To visualize the change of calcium ions in behaving subjects, I will inject engineered Adeno-associated virus (AAV) and express calcium indicator in the neurons. With this approach, I expected to have a clear view of the potential involvement of each brain areas in different time points of the saccade tasks. Furthermore, I will use either optogenetic excitation or inhibition methods to test the causal involvement of those brain areas in saccade generation (Fig 2c). This will help me to resolve the causal relationship of cortical and subcortical interactions involved in saccade generation.
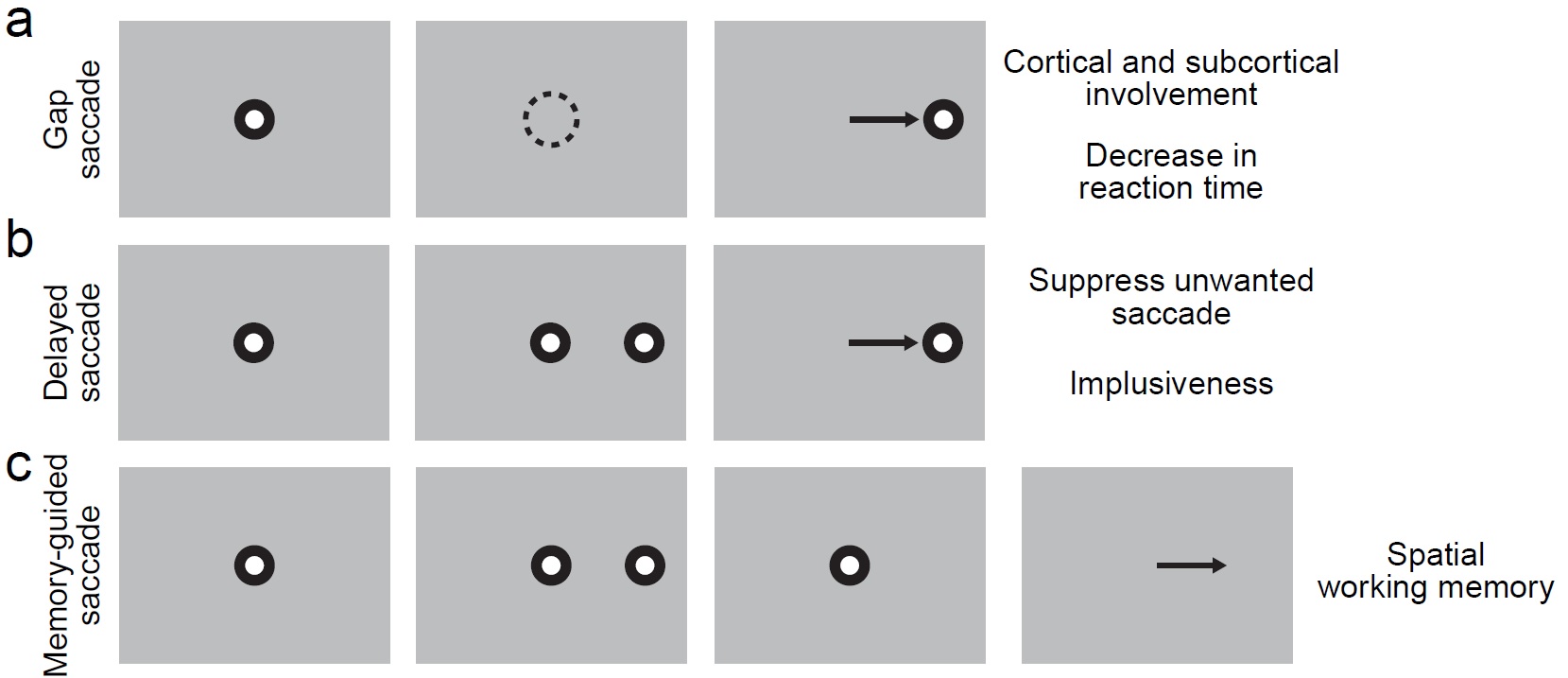
Fig 1: Example saccade tasks for marmosets with real-time monitoring of eye movement. (a) Gap saccade task will be used to access the change of saccade reaction time. A spot is shown at the center of the monitor indicating the subject to fixate and initiate the trial in the left most figure. Then, the subject will be trained to maintain fixation within an invisible window (dashed circle) while the fixation spot is turned off as shown second left figure. As the last figure showed, the subject will make a saccade to the target when the target appears. This task has been shown to have decreased saccade reaction time compared with regular visually guided saccade task. However, the mechanism is still not clear. (b) Delayed saccade task can be used as a good indication of the impulsiveness of the subject. The first step for delayed saccade task is the same as for gap saccade task (left most figure.) While the fixation spot is still on, the target will pop-out in the periphery (second left figure.) During this period of time, the subject needs to maintain fixation at the center and don’t to look at the target. At the end of the trial, the fixation spot is turned off and the subject is allowed to make a saccade to the target as indicated in the last figure. (c) Memory-guided saccade task can be used as a simple way to access spatial working memory ability. The left most figure is again showing the first step with fixation spot. The second figure from the left is showing while the fixation spot is still present, there will be a short flash of the target (typically 50 ms.) The subject need to keep fixation for a period of time and memorize the target location (second right figure.) After fixation spot is removed, the subject is allowed to answer where it saw the target by saccading to the target location on a blank screen.
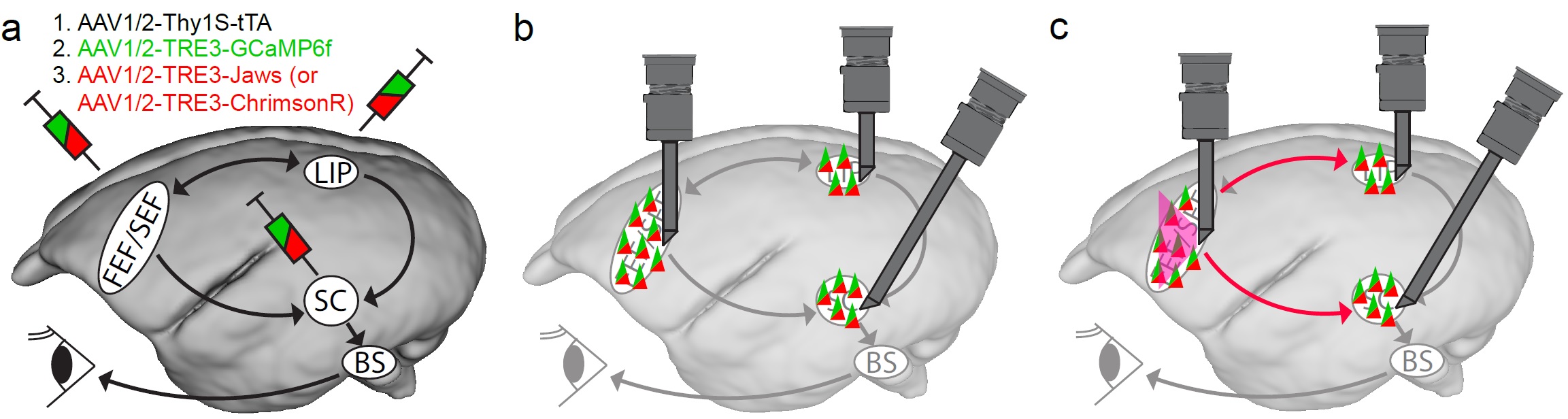
Fig 2: Resolving the causal relationship of brain areas involving in saccade generation. (a) Injecting engineered AAV in the brain areas critical for saccade generation. Here is showing the potential saccade generation diagram in marmoset where the cortical brain areas mentioned above send the saccade command to the SC and further to the brain stem saccade circuit (BS). The BS then use this command to generate a precise saccadic eye movement. To monitor neuronal activities and gain control of the circuit, I will inject engineered AAV to express of our target proteins, GCaMP6f (a genetically encoded calcium indicator) for calcium activity indication, and ChrimsonR (red-light drivable channelrhodopsin) or Jaws (red-light drivable halorhodopsin) for exiting or inhibiting specific brain region as showed in the figure, respectively. The injected AAVs will include an enhancing system, the tetracycline-controlled transcriptional activation (TeT) system, which the first AAV, AAV1/2-Thy1S-tTA will produce tetracycline and bind to the promotor, TRE, of the other AAVs to enhance the production of the target protein. (b) After sufficient GCaMP6f expression, I will use multiple endoscope systems (in grey) to monitor the neuronal activity in all the critical brain areas simultaneously while the marmosets perform saccadic tasks (Fig 1). (c) We will also be able to enhance or inhibit specific brain area with the same system. For example, we can shine red light to activate or inhibit the FEF as showed in the figure in red lines with precise timing during saccade tasks. At the meantime, we can monitor the other brain areas and see of the neuronal activity changes due to such manipulation. We will also correlate the behavioral change of the eye movement to the manipulation to establish the causal link between specific brain area to saccade generation.
Biography
Chih-Yang Chen went to Germany and obtained his PhD in Neuroscience form the University of Tuebingen in 2017. He later moved to Kyoto and undertook postdoctoral training with JSPS Postdoctoral Fellowship in Prof. Tadashi Isa’s group in Kyoto University until 2020. He was than appointed as an Assistant Professor in 2020 in the Institute for the Advanced Study of Human Biology (ASHBi) of Kyoto University.
Publications
Chen, C. -Y., Hoffmann, K. P., Distler, C., & Hafed, Z. M. (2019). The Foveal Visual Representation of the Primate Superior Colliculus. Current Biology, Vol. 29, No. 13, pp. 2109-2119
Chen, C. -Y., Sonnenberg, L., Weller, S., Witschel, T., & Hafed, Z. M. (2018). Spatial frequency sensitivity in macaque midbrain. Nature Communications. doi: 10.1038/s41467-018-05302-5
Chen, C. -Y. and Hafed, Z. M. (2017). A neural locus for spatial-frequency specific saccadic suppression in visual-motor neurons of the primate superior colliculus. Journal of Neurophysiology, Vol. 117, No. 4, pp. 1657-1673
Hafed, Z. M. and Chen, C. -Y. (2016). Sharper, stronger, faster upper visual field representation in primate superior colliculus. Current Biology, Vol. 26, No. 13, pp. 1647-1658
Chen, C. -Y., Ignashchenkova, A., Thier, P., and Hafed, Z. M. (2015). Neuronal response gain enhancement prior to microsaccades. Current Biology, Vol. 25, No. 16, pp. 2065-2074.
Chen, C. -Y. and Hafed, Z. M. (2013). Postmicrosaccadic enhancement of slow eye movements. The Journal of Neuroscience, Vol. 33, No. 12, pp. 5375-5386.
Awards
Annual Attempto Prize (for Tübingen-based research published), the Attempto Foundation in Germany (2015);
Annual Trainee Professional Development Awards, Society of Neuroscience (2016);
The Excellent Research Award, ASHBi Retreat 2020 (2020)
Research Group
Isa GroupJoined
Apr. 1, 2020
