Research


Multi-cellular self-organisation
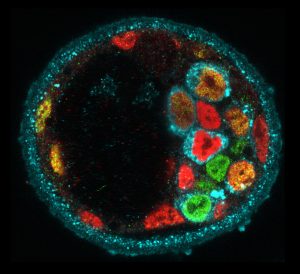
Molecular heterogeneity during mouse blastocyst patterning. Cells expressing Nanog (green), Gata6 (red) or Serpinh1 (blue).
We aim to understand the principles underlying the self-organisation of multicellular systems, i.e. the emergence of functional order. There are various interactions across the scales in multicellular systems, from molecules to organelles, cells, and tissues. We identify the critical interactions one by one to reveal how a fertilised egg develops into a complex shape and pattern.
Self-organisation principle
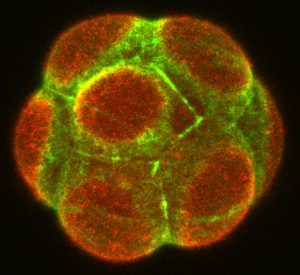
Symmetry breaking in the mouse embryo at the 8-cell stage (the emerging apical domain marked with Ezrin, red; Actin, green).
Mammalian eggs lack polarity and symmetry is broken during early embryogenesis. Our studies revealed that morphogenesis and gene expression are highly variable between embryos and cells during this period (Dietrich et al. 2007 Development; Ohnishi et al. 2014 Nat Cell Biol; Niwayama et al. 2019 Dev Cell). Determining how early embryos develop with reproducible form despite such variability remains a fundamental question in mammalian development. We established a multi-disciplinary framework integrating biology, physics and mathematics, which showed that feedback interactions between cell and tissue mechanics (contractility, adhesion, geometry and pressure), polarity, and fate robustly control size and inside-outside patterning of the early mouse embryo (Korotkevich et al. 2017 Dev Cell; Maître et al. 2016 Nature; Chan et al. 2019 Nature). We will build on our mechanistic understanding, and integrate the self-organisation model to tissue-tissue and embryo-uterus interactions that were revealed by our recent study (Ichikawa, Zhang, Panavaite et al. 2022 Dev Cell; Bondarenko et al. 2023 EMBO J).
Embryo size control
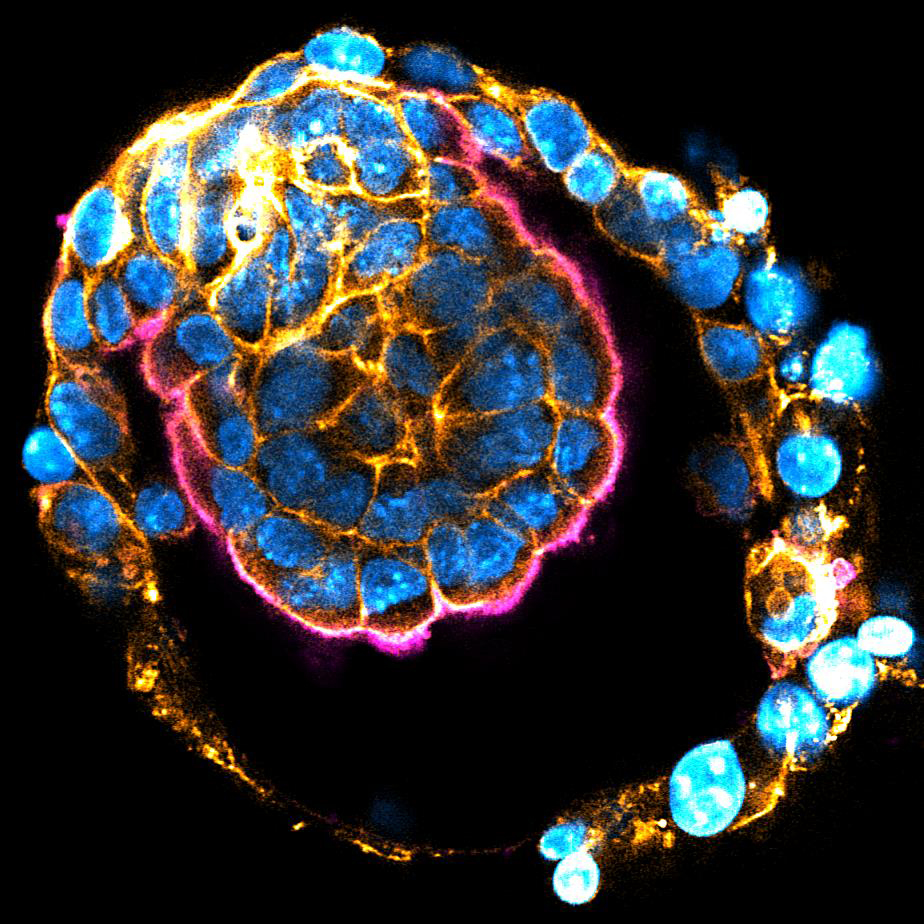
Mouse implanting embryo. Nucleus (blue), Actin (orange), Ezrin (magenta)
The size of embryos and their organs is precisely controlled in each species, despite the inherent variabilities observed during morphogenesis and growth. The previously reported mechanism for embryo size control (Chan et al. 2019 Nature) may work only when fluid cavity expansion is substantially faster than tissue growth, as in pre-implantation embryos. However, the mechanism underlying the remarkable precision in post-implantation growth remains unknown. Using our newly developed ex vivo culture system (Ichikawa, Zhang, Panavaite et al. 2022 Dev Cell; Bondarenko et al. 2023 EMBO J), we will dissect the dynamic changes in cell and tissue growth. We aim to understand how the size of tissues and embryos are sensed, and how this feeds back into the system to control cellular growth dynamics.
