Research Overview
Physiological and Genetic Approaches to Understanding and Controlling Anxiety Circuits in Macaque Monkeys
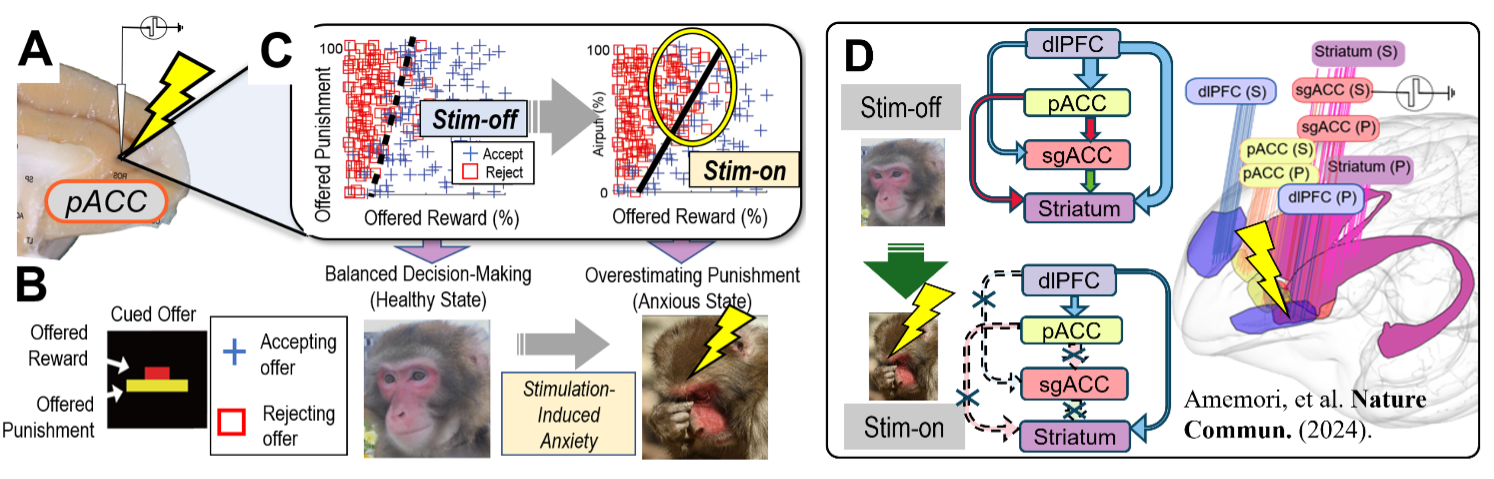
We aim to elucidate the neural circuit mechanisms underlying psychiatric disorders such as anxiety and depression, and to develop effective methods to control these circuits. To quantitatively examine how anxiety influences decision-making, we have adopted a psychological framework known as the “approach-avoidance conflict.” This task involves simultaneously presenting subjects with rewards and punishments, compelling them to decide whether to accept or avoid these combined outcomes. We implemented this decision-making task in macaque monkeys and quantitatively assessed their degree of pessimistic decision-making based on their behavioral choices (Fig. 1A, B). Furthermore, by applying localized electrical stimulation to the cingulate cortex and the striatum, we observed a significant increase in avoidance behavior, indicating enhanced pessimistic decision-making (Fig. 1C). Injection of tracer viruses into these effective stimulation sites revealed a specific neural projection pathway from the pregenual anterior cingulate cortex (pACC) to the striosome compartments of the striatum, suggesting that this pathway mediates the observed behavioral bias.
How Does Pessimistic Decision-Making Arise?
What are the neural mechanisms responsible for generating pessimistic decision-making? To address this question, we defined a widespread neural network centered on the prefrontal cortex, termed the “primate anxiety network.” By simultaneously recording neural activity across multiple regions within this network, we successfully captured the neural dynamics underlying conflicts between cognitive and emotional processes (Fig. 1D). Specifically, we observed diminished top-down regulation from the prefrontal cortex during stimulation-induced pessimistic states. Recent human fMRI studies have also reported similar patterns of neural activity, suggesting that the primate anxiety network may represent a common neural substrate underlying anxiety across primate species. Based on these physiological findings, we are currently pursuing the following research topic.
Research Topic 1: Interareal Interactions between Cognitive and Limbic Systems in Primates
The primate brain is substantially larger and more complex than that of rodents, and interactions between cortical and subcortical regions represent a significant evolutionary advancement. Within the anxiety network, we hypothesize that synchronized neural oscillations play a crucial role in interareal signal transmission. To test this hypothesis, we have developed experimental protocols combining microstimulation, multi-site simultaneous neural recordings, and functional MRI (fMRI). This integrated approach will allow us to clarify how synchronized neural oscillations contribute to information integration across brain regions within the anxiety network (Fig. 1).
How Do the Circuits Relate to Anxiety and Depressive Disorders?
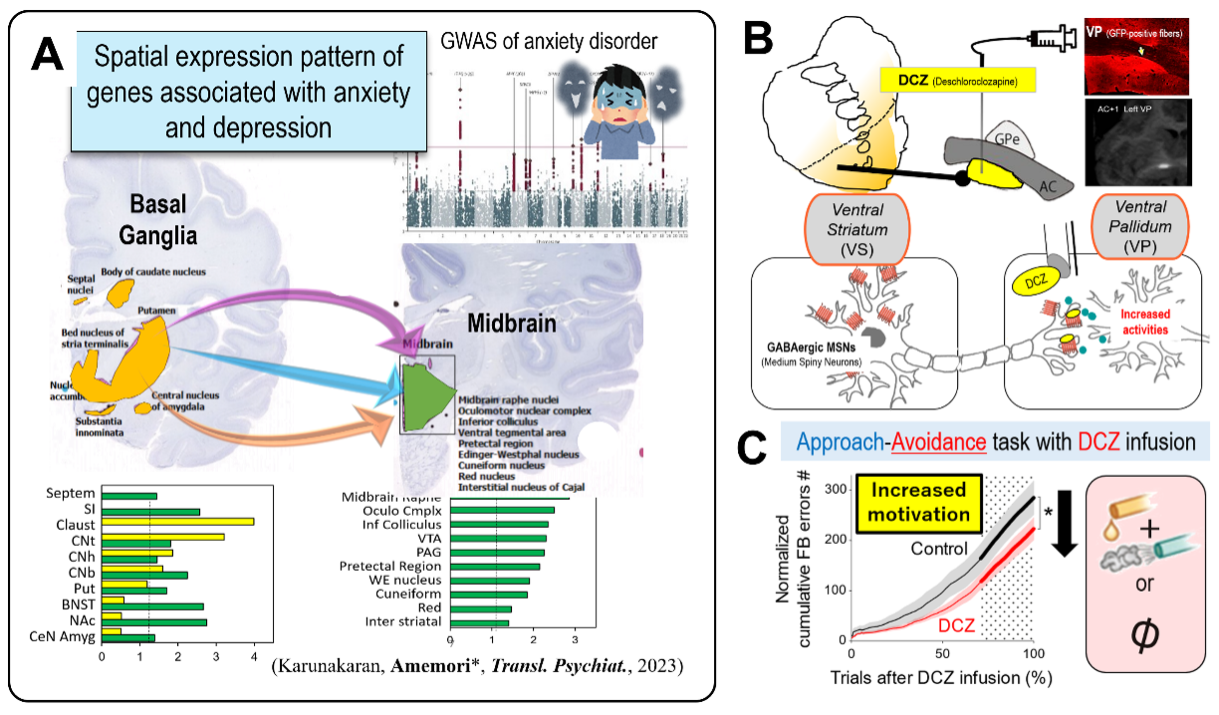
How do the neural circuits underlying pessimistic decision-making relate to anxiety disorders and depression? To address this question, we first analyzed the brain-wide expression patterns of genes associated with anxiety disorders. Our analysis revealed significantly elevated expression levels of these anxiety-related genes within multiple nuclei of the basal ganglia and midbrain (Fig. 2A). This finding suggests a genetic basis linking anxiety and depressive disorders to the neural circuits that constitute the primate anxiety network. Based on this evidence, we are currently pursuing the following research project.
Research Topic 2: Genetic Approaches for Identifying Anxiety Circuits in Primates
In this project, we focus on cortico–basal ganglia–midbrain pathways expressing anxiety-related genes, aiming to elucidate the mechanisms underlying depression and anxiety disorders. Using chemogenetics, we perform targeted manipulations of these circuits in macaque monkeys to investigate how pessimistic decision-making arises. Through selective manipulation of specific neural pathways, we have discovered that the ventral pallidal pathway significantly contributes to reduced motivation (Fig. 2B). These findings identify the ventral pallidum as a promising therapeutic target for symptoms such as apathy and avolition, commonly observed in depression and negative symptoms of schizophrenia.
Publications
Amemori, S., Graybiel, A., & Amemori, K. (2024). Cingulate microstimulation induces negative decision-making via reduced top-down influence on primate fronto-cingulo-striatal network. Nature Communications, 15(1), Article 4201. https://doi.org/10.1038/s41467-024-48375-1
Karunakaran, K., & Amemori, K. (2023). Spatiotemporal expression patterns of anxiety disorder-associated genes. Translational Psychiatry, 13(1), Article 385. https://doi.org/10.1038/s41398-023-02693-y
Ironside, M*., Amemori, K*., McGrath, C., Pedersen, M., Kang, M., Amemori, S., … Pizzagalli, D. (2020). Approach-avoidance conflict in major depressive disorder: Congruent neural findings in humans and nonhuman primates. Biological Psychiatry, 87(5), 399–408. https://doi.org/10.1016/j.biopsych.2019.08.022
Schwerdt, H., Amemori, K., Gibson, D., Stanwicks, L., Yoshida, T., Bichot, N., … Graybiel, A. (2020). Dopamine and beta-band oscillations differentially link to striatal value and motor control. Science Advances, 6(39), Article eabb9226. https://doi.org/10.1126/sciadv.abb9226
Amemori, K*., Amemori, S*., Gibson, D., & Graybiel, A. (2018). Striatal microstimulation induces persistent and repetitive negative decision-making predicted by striatal beta-band oscillation. Neuron, 99(4), 829+. https://doi.org/10.1016/j.neuron.2018.07.022
Friedman, A., Homma, D., Bloem, B., Gibb, L., Amemori, K., Hu, D., … Graybiel, A. (2017). Chronic stress alters striosome-circuit dynamics, leading to aberrant decision-making. Cell, 171(5), 1191+. https://doi.org/10.1016/j.cell.2017.10.017
Desrochers, T., Amemori, K., & Graybiel, A. (2015). Habit learning by naive macaques is marked by response sharpening of striatal neurons representing the cost and outcome of acquired action sequences. Neuron, 87(4), 853–868. https://doi.org/10.1016/j.neuron.2015.07.019
Friedman, A., Homma, D., Gibb, L., Amemori, K., Rubin, S., Hood, A., … Graybiel, A. (2015). A corticostriatal path targeting striosomes controls decision-making under conflict. Cell, 161(6), 1320–1333. https://doi.org/10.1016/j.cell.2015.04.049
Amemori, K., Amemori, S., & Graybiel, A. (2015). Motivation and affective judgments differentially recruit neurons in the primate dorsolateral prefrontal and anterior cingulate cortex. Journal of Neuroscience, 35(5), 1939–1953. https://doi.org/10.1523/JNEUROSCI.1731-14.2015
Amemori, K., & Graybiel, A. (2012). Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nature Neuroscience, 15(5), 776–785. https://doi.org/10.1038/nn.3088
*equal contribution