Japanese Researchers Successfully Induce Primate Oocytes In the Lab
10.15252/embj.2022112962
The many types of cells in the human body are produced through the process of differentiation, in which stem cells are converted to more specialized types. Currently, it is challenging for researchers to control the differentiation of stem cells in the lab (in vitro). Of particular interest are oocytes, which are female germ cells that develop into eggs. Understanding their development could have far-ranging impacts, from infertility treatment to conservation of endangered species. A new study by a Japanese team of researchers led by Dr. Mitinori Saitou has successfully induced meiotic (dividing) oocytes from the embryonic stem cells of cynomolgus monkeys, which share many physiological traits with humans. By establishing a culture method for inducing the differentiation of meiotic oocytes, the researchers aimed to shed light on germ cell development in both humans and other primates. The study’s findings were published in the March 2023 issue of The EMBO Journal.
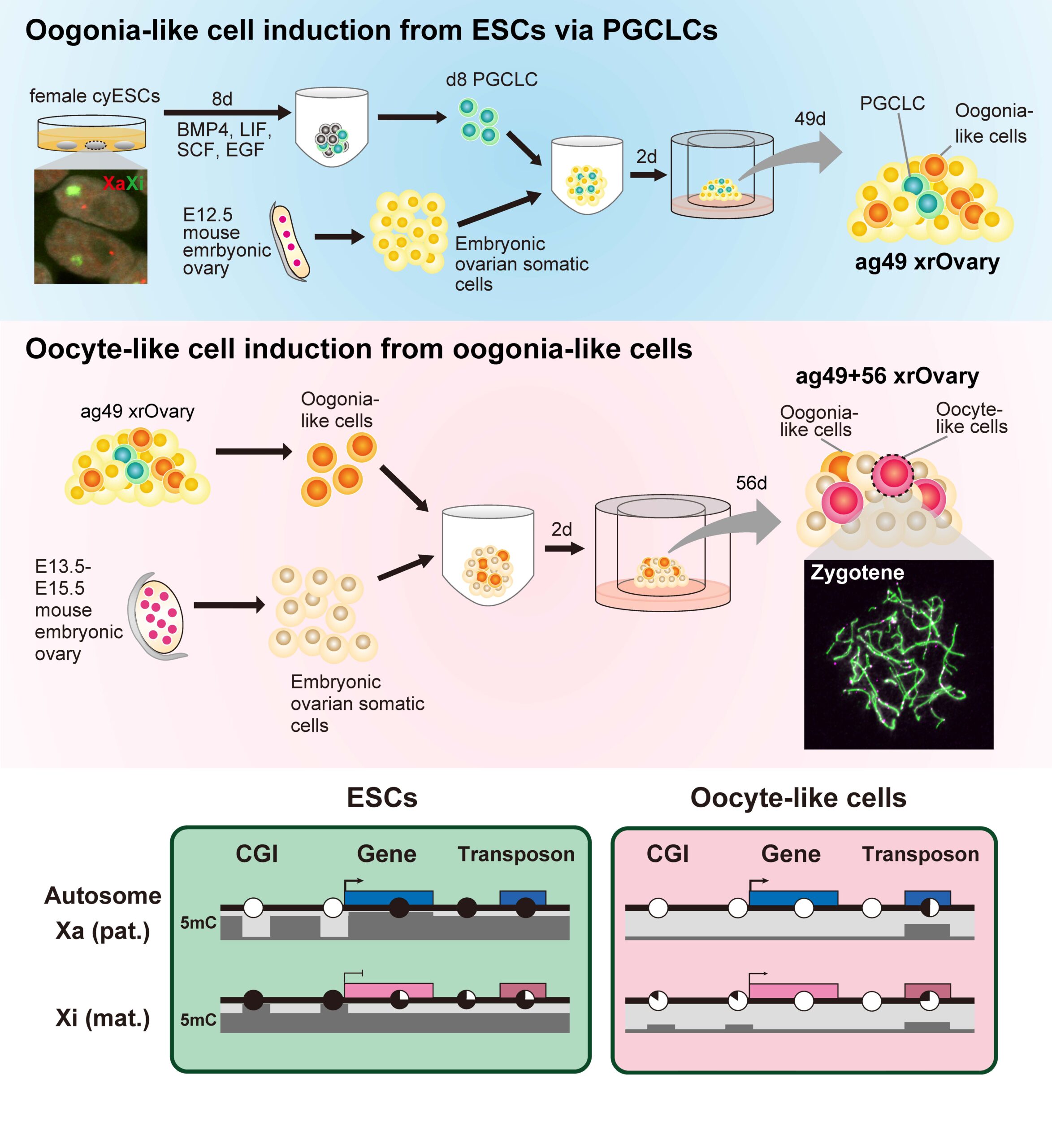
The team previously reported conditions for inducing oogonia, the precursors of oocytes, by aggregating human primordial germ cell-like cells (hPGCLCs) with cells from the ovaries of female mouse embryos and then culturing them under air–liquid interface conditions1. Similarly, PGCLCs from cynomolgus monkey were induced to differentiate into oogonia but did not progress to meiotic oocytes. To overcome this hurdle, the induced oogonia were isolated and re-aggregated with somatic cells from the ovaries of female mouse embryos and cultured again.
Under these new culture conditions, the cynomolgus monkey oogonia were successfully induced to differentiate into meiotic oocytes, but their development stopped at the second stage of meiosis. Single-cell transcriptome analysis showed that the transcriptomic dynamics of the oocytes in vitro (in the lab) were similar to those of oocytes in vivo (in our body). The researchers also identified differences in gene expression between the in vitro and in vivo oocytes, which suggested a bottleneck for in vitro oocyte development that might lead to the arrest of meiosis in vitro.
Furthermore, by performing whole-genome methylome analysis, the authors found that the induced oocytes were involved in the genome-wide demethylation process in vitro, as seen in mouse and human female germ cell development. They also noticed that demethylation behaved differently in paternally and maternally-derived X chromosomes. These unique methylation dynamics were also found in human oogonia induced in vitro, suggesting that the mechanisms underlying female germ cell development may be the same across primate species. Thus, this culture system might be useful as a model of the primate germ cell differentiation process.
Asked about the potential impact of their study, the authors said that their method of reconstituting multiple steps in the development of female germ cells may help to clarify the molecular mechanisms of primate oocyte development and could one day contribute to the treatment of impaired oocyte development in reproductive medicine. First author Dr. Sayuri Gyobu-Motani says, “We hope that that our culture system can aid in the conservation of endangered species and the creation of in vitro oocyte induction systems for other mammalian species with long lifespans.”
Paper Information
Gyobu-Motani, S., Yabuta, Y., Mizuta, K., Katou, Y., Okamoto, I., Kawasaki, M., Kitamura, A., Tsukiyama, T., Iwatani, C., Tsuchiya, H., Tsujimura, T., Yamamoto, T., Nakamura, T. and Saitou, M. (2023) Induction of fetal meiotic oocytes from embryonic stem cells in cynomolgus monkeys. EMBO JOURNAL,10.15252/embj.2022112962
Reference
1.Yamashiro et al., DOI: 10.1126/science.aat1674