Kyoto scientists zoom in on kidney damage
New cell-imaging technique offers insight into how kidney disease develops
Our kidneys need a continuous blood supply to function properly. The disruption in blood flow, such as from blood vessel blockage, can injure the kidneys as a result of oxygen deprivation. In addition, the reperfusion worsens the damage by triggering inflammation and scarring, which could lead to a lifetime of poor health.
Treating this kind of kidney damage requires a full understanding of injury and disease. From animal studies, researchers know that as disease progresses, the kidney’s filtration units (or nephrons) change the way they generate and use energy. However, research has been limited by the lack of technology to study energy changes at a cellular level in living tissue within intact kidneys.
Now a research team led by the Institute for the Advanced Study of Human Biology at Kyoto University has developed such a method to directly visualize and quantify chemical energy at single-cell resolution in nephrons inside the kidneys of live mice. The group used the new method to study energy levels in different parts of nephrons in real time and in three dimensions, during and after a simulated injury.
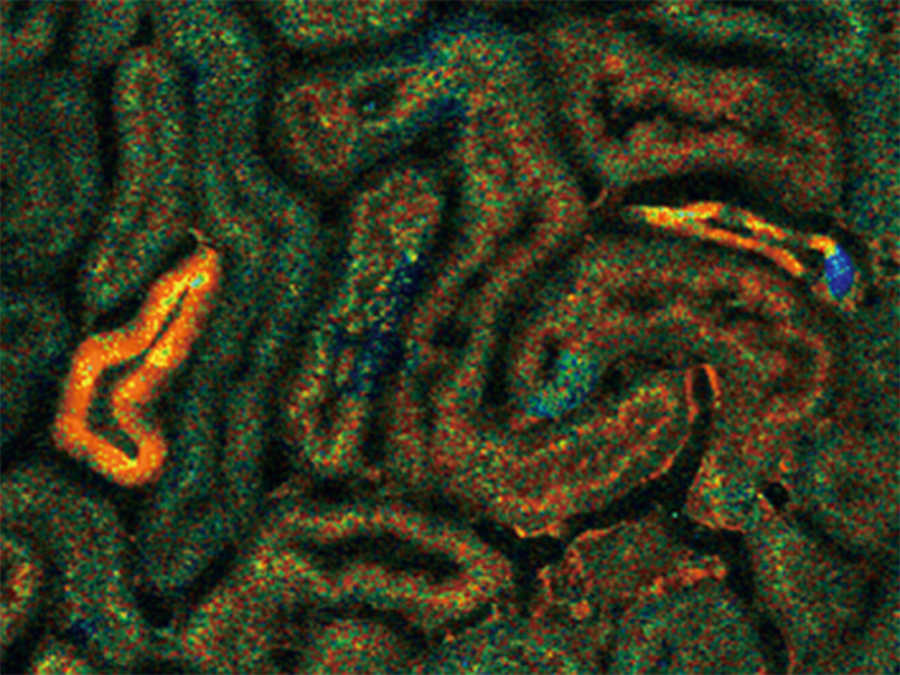
The imaging technique works by detecting the molecule adenosine triphosphate, or ATP, which is consumed for reabsorption of essential nutrients and electrolytes in kidneys. ATP is made mainly in special structures called mitochondria, which are the powerhouses of the cell.
“ATP represents the currency of cellular energy,” lead author Shinya Yamamoto of Kyoto University explains. “We know that certain parts of the kidney’s nephrons use up large amounts of ATP during their normal operation but until now, we had no way of seeing what was happening to ATP during kidney damage.”
The team used genetically modified mice whose cells contained biosensor molecules that glowed with fluorescent light in the presence of ATP. With the help of a two-photon microscope, which allowed high-speed and noninvasive imaging, the researchers measured the amounts of ATP in different regions of nephrons below the surface of exposed kidneys. They monitored changes in ATP level after the blood vessels entering the kidney were clamped shut with a clip for up to an hour and then unclamped to restore the blood supply.
After the blood supply was stopped, the ATP levels fell to a minimum within two minutes in a region near the start of the nephron that is normally intensely active, called the proximal tubule. But in a region further along the nephron — the distal tubule — the ATP concentration fell much more slowly over an hour.
When the blood flow was restarted, ATP levels in the distal tubules quickly recovered. In contrast, ATP levels in the proximal tubules increased slowly and did not reach their original amounts. High-magnification electronic microscopy revealed that in cells of the proximal tubules, but not the distal tubules, the mitochondria had shattered into many fragments. These results confirmed a theory that these two different parts of the nephron make and use ATP in different ways.
The team also discovered that longer durations of blood flow stoppage were linked to lower rates and levels of ATP recovery in the proximal tubules, as well as more severe kidney scarring two weeks later. These findings prompted the researchers to suggest that early ATP recovery in the proximal tubules is predictive of the prognosis, or overall outcome, of kidney disease.
Furthermore, slight cooling of the mice and kidneys during the blood vessel clamping step conferred a protective effect. Compared with kidneys in the experiments without cooling, the kidneys that had been cooled showed not only faster and fuller ATP recovery in the proximal tubules, but also less scarring two weeks later. This observation supports the common practice of cooling kidney transplants or kidneys undergoing cancer-removal surgery to minimize damage from temporary blood flow interruption, the researchers comment.
Group leader Motoko Yanagita of Kyoto University says: “This revolutionary ATP imaging technique is improving our understanding of cellular events in the diseased kidney. We could also apply this technology to other organs, such as the brain and heart, and address many questions about diseases where the body’s use of energy is important.”
Paper Information
Shinya Yamamoto, Masamichi Yamamoto, Jin Nakamura, Akiko Mii, Shigenori Yamamoto, Masahiro Takahashi, Keiichi Kaneko, Eiichiro Uchino, Yuki Sato, Shingo Fukuma, Hiromi Imamura, Michiyuki Matsuda, Motoko Yanagita (2020). Spatiotemporal ATP dynamics during acute kidney injury predicts renal prognosis, Journal of the American Society of Nephrology, DOI: https://doi.org/10.1681/ASN.2020050580
Writing: Nano Pico Science