Motoko Yanagita
| Position | Professor |
|---|---|
| Group name | Yanagita Group |
| Research Field | Nephrology |
Research Overview
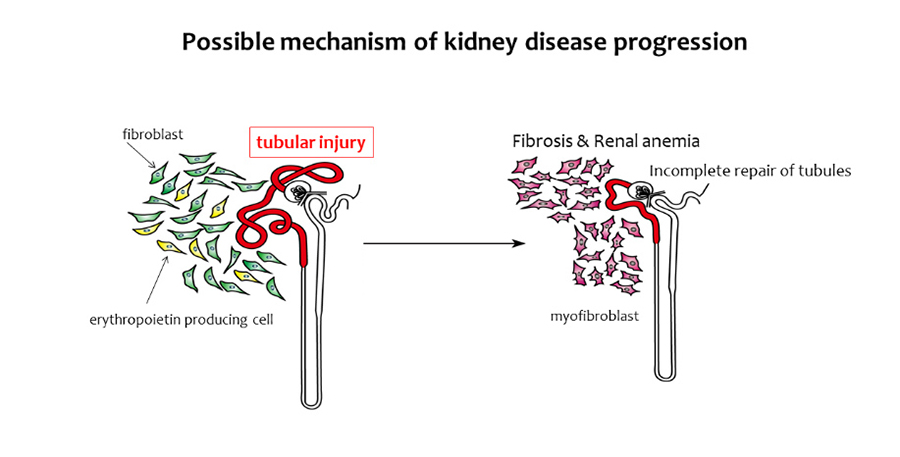
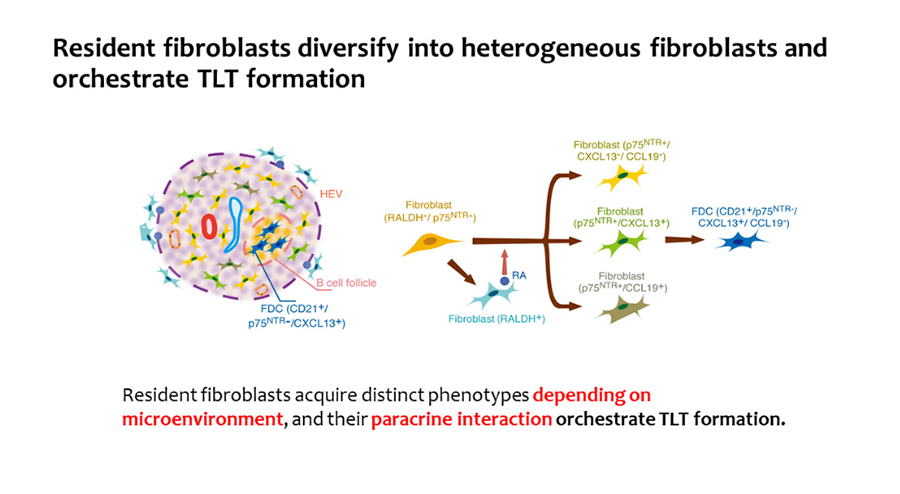
More than 320,000 patients with renal failure are undergoing hemodialysis in Japan. We are studying the regenerative ability of the kidney, which can be linked to the treatment of kidney disease. We have identified the cell populations responsible for kidney injury and repair, and have revealed the intercellular crosstalk which controls the development and progression of kidney disease. Fibrosis and renal anemia are the hallmarks of advanced kidney diseases. Previously we have found that these two features are caused by the fibroblasts-to-myofibroblasts transdifferentiation in the kidney. In addition, we have shown that the transdifferentiation of fibroblasts is induced by renal tubular damage, and that fibroblasts acquire the ability to produce retinoic acids during the transdifferentiation, which promotes tubular repair. Furthermore, we have shown that renal tubule has self-repairing ability, although the ability is not sufficient if the damage is severe. Elderly people have incomplete renal repair capacity, but the reason of incomplete repair was not clarified. We have shown that tertiary lymphoid tissues (TLTs) are formed in aged injured kidneys, and sustained inflammation due to TLT formation delays kidney regeneration. Interestingly, fibroblasts in the kidney acquire distinct phenotypes which promotes TLT formation in aged kidneys. In this project, we will extend our current understanding of the mechanisms of kidney injury with monkey models and human tissues.
Publications
Toriu, N., Sato, Y., Kamimura, H., Yoshikawa, T., Tanaka, M., Yamamoto, S., Fukuma, S., Hattori, M., Terai, S., & Yanagita, M. (2025). Aligning cellular and molecular components in age-dependent tertiary lymphoid tissues of kidney and liver. PLoS ONE, 20(2), e0311193. https://doi.org/10.1371/journal.pone.0311193
Kitai, Y., Toriu, N., Yoshikawa, T., Sahara, Y., Kinjo, S., Shimizu, Y., Sato, Y., Oguchi, A., Yamada, R., Kondo, M., Uchino, E., Taniguchi, K., Arai, H., Sasako, T., Haga, H., Fukuma, S., Kubota, N., Kadowaki, T., Takasato, M., Murakawa, Y., & Yanagita, M. (2025). Female sex hormones inversely regulate acute kidney disease susceptibility throughout life. Kidney International, 107(1), 68–83. https://doi.org/10.1016/j.kint.2024.08.034
Takahashi, M., Yamamoto, S., Yamamoto, S., Okubo, A., Nakagawa, Y., Kuwahara, K., Matsusaka, T., Fukuma, S., Yamamoto, M., Matsuda, M., & Yanagita, M. (2024). ATP dynamics as a predictor of future podocyte structure and function after acute ischemic kidney injury in female mice. Nature Communications, 15(1), 9977. https://doi.org/10.1038/s41467-024-54222-0
Yamamoto, S., Yamamoto, S., Takahashi, M., Mii, A., Okubo, A., Toriu, N., Nakagawa, S., Abe, T., Fukuma, S., Imamura, H., Yamamoto, M., & Yanagita, M. (2024). Visualization of intracellular ATP dynamics in different nephron segments under pathophysiological conditions using the kidney slice culture system. Kidney International. Advance online publication. https://doi.org/10.1016/j.kint.2024.05.028
Oguchi, A., Suzuki, A., Komatsu, S., Yoshitomi, H., Bhagat, S., Son, R., Bonnal, R. J. P., Kojima, S., Koido, M., Takeuchi, K., ... Yanagita, M. (2024). An atlas of transcribed enhancers across helper T cell diversity for decoding human diseases. Science, 385(6704), eadd8394. https://doi.org/10.1126/science.add8394
Yoshikawa, T., Oguchi, A., Toriu, N., Sato, Y., Kobayashi, T., Ogawa, O., Haga, H., Sakurai, S., Yamamoto, T., Murakawa, Y., & Yanagita, M. (2023). Tertiary lymphoid tissues are microenvironments with intensive interactions between immune cells and proinflammatory parenchymal cells in aged kidneys. Journal of the American Society of Nephrology, 34(10), 1687–1708. https://doi.org/10.1681/ASN.0000000000000202
Sato, Y., Oguchi, A., Fukushima, Y., Masuda, K., Toriu, N., Taniguchi, K., Yoshikawa, T., Cui, X., Kondo, M., Hosoi, T., ... Yanagita, M. (2022). CD153/CD30 signalling promotes age-dependent tertiary lymphoid tissue expansion and kidney injury. Journal of Clinical Investigation, 132(2), e146071. https://doi.org/10.1172/JCI146071
Lee, Y. H., Sato, Y., Saito, M., Fukuma, S., Saito, M., Yamamoto, S., Komatsuda, A., Fujiyama, N., Satoh, S., Lee, S.-H., ... Yanagita, M. (2022). Advanced tertiary lymphoid tissues in protocol biopsies are associated with progressive graft dysfunction in kidney transplant recipients. Journal of the American Society of Nephrology, 33(1), 186–200. https://doi.org/10.1681/ASN.2021050715
Kaneko, K., Sato, Y., Uchino, E., Toriu, N., Shigeta, M., Kiyonari, H., Endo, S., Fukuma, S., & Yanagita, M. (2022). Lineage tracing analysis defines erythropoietin-producing cells as a distinct subpopulation of resident fibroblasts with unique behaviors. Kidney International, 102(2), 280–292. https://doi.org/10.1016/j.kint.2022.04.026
Yamamoto, S., Yamamoto, M., Nakamura, J., Mii, A., Yamamoto, S., Takahashi, M., Kaneko, K., Uchino, E., Sato, Y., Fukuma, S., ... Yanagita, M. (2020). Spatiotemporal ATP dynamics during acute kidney injury predicts renal prognosis. Journal of the American Society of Nephrology, 31(12), 2855–2869. https://doi.org/10.1681/ASN.2020050580